

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM160918 US9107923, 7::US9107923, 8
SMILES: Cc1cc(Oc2ncccc2C(F)(F)F)ccc1-c1c(C)c(=O)[nH]c(=O)n1C
InChI Key: InChIKey=AKQXQLUNFKDZBN-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160918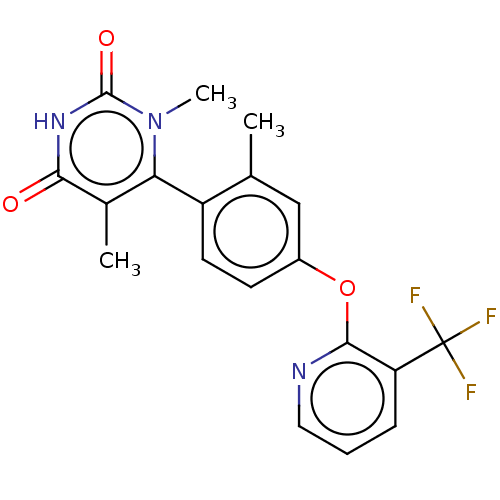 (US11014909, Example 8 | US9107923, 7 | US9107923, ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Partial agonist activity at human D1R | J Med Chem 62: 128-140 (2019) Article DOI: 10.1021/acs.jmedchem.8b01767 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160918 (US11014909, Example 8 | US9107923, 7 | US9107923, ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | 8.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description D1 binding assays were performed using over-expressing LTK human cell lines. To determine basic assay parameters, ligand concentrations were determin... | US Patent US11014909 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160918 (US11014909, Example 8 | US9107923, 7 | US9107923, ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | 8.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., Dif... | US Patent US9107923 (2015) BindingDB Entry DOI: 10.7270/Q2C24V5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160918 (US11014909, Example 8 | US9107923, 7 | US9107923, ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., Dif... | US Patent US9107923 (2015) BindingDB Entry DOI: 10.7270/Q2C24V5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160918 (US11014909, Example 8 | US9107923, 7 | US9107923, ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description D1 binding assays were performed using over-expressing LTK human cell lines. To determine basic assay parameters, ligand concentrations were determin... | US Patent US11014909 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||