 Found 18 hits for monomerid = 164883
Found 18 hits for monomerid = 164883 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM164883
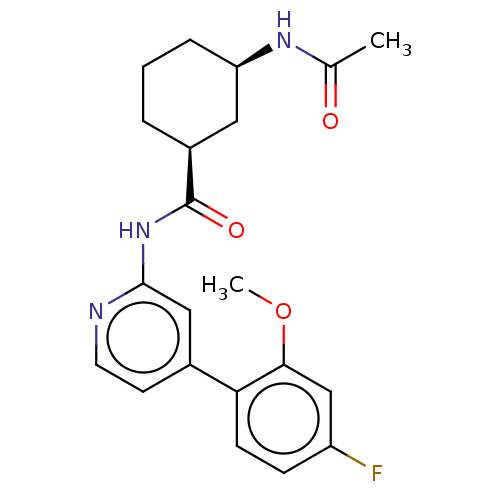
(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
In vitro kinase assay analysis may be performed using standard techniques described in the art. These techniques are also used by commercial services... |
US Patent US9067888 (2015)
BindingDB Entry DOI: 10.7270/Q2CR5S4T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 3
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 219 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
In vitro kinase assay analysis may be performed using standard techniques described in the art. These techniques are also used by commercial services... |
US Patent US9067888 (2015)
BindingDB Entry DOI: 10.7270/Q2CR5S4T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
In vitro kinase assay analysis may be performed using standard techniques described in the art. These techniques are also used by commercial services... |
US Patent US9067888 (2015)
BindingDB Entry DOI: 10.7270/Q2CR5S4T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
In vitro kinase assay analysis may be performed using standard techniques described in the art. These techniques are also used by commercial services... |
US Patent US9067888 (2015)
BindingDB Entry DOI: 10.7270/Q2CR5S4T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
In vitro kinase assay analysis may be performed using standard techniques described in the art. These techniques are also used by commercial services... |
US Patent US9067888 (2015)
BindingDB Entry DOI: 10.7270/Q2CR5S4T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
In vitro kinase assay analysis may be performed using standard techniques described in the art. These techniques are also used by commercial services... |
US Patent US9067888 (2015)
BindingDB Entry DOI: 10.7270/Q2CR5S4T |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
In vitro kinase assay analysis may be performed using standard techniques described in the art. These techniques are also used by commercial services... |
US Patent US9067888 (2015)
BindingDB Entry DOI: 10.7270/Q2CR5S4T |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 903 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
In vitro kinase assay analysis may be performed using standard techniques described in the art. These techniques are also used by commercial services... |
US Patent US9067888 (2015)
BindingDB Entry DOI: 10.7270/Q2CR5S4T |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 1
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
In vitro kinase assay analysis may be performed using standard techniques described in the art. These techniques are also used by commercial services... |
US Patent US9067888 (2015)
BindingDB Entry DOI: 10.7270/Q2CR5S4T |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) using cdk7tide peptide as substrate |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His/GST-tagged CDK2/Cyclin A expressed in baculovirus infected Sf9 cells using Histone H1 as substrate after 80 mins ... |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His/GST-tagged CDK5/p35 expressed in baculovirus infected Sf9 insect cells using Rb-CTF as substrate after 80 mins in... |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK1/cyclin B expressed in baculovirus infected Sf9 insect cells after 80 mins in presence of [33P]ATP by microbeta s... |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 3/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK3/cyclin E expressed in baculovirus infected Sf9 insect cells after 80 mins in presence of [33P]ATP by microbeta s... |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 168 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
In vitro kinase assay analysis may be performed using standard techniques described in the art. These techniques are also used by commercial services... |
US Patent US9067888 (2015)
BindingDB Entry DOI: 10.7270/Q2CR5S4T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data