 Found 17 hits for monomerid = 16596
Found 17 hits for monomerid = 16596 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Collagenase 3
(Homo sapiens (Human)) | BDBM16596
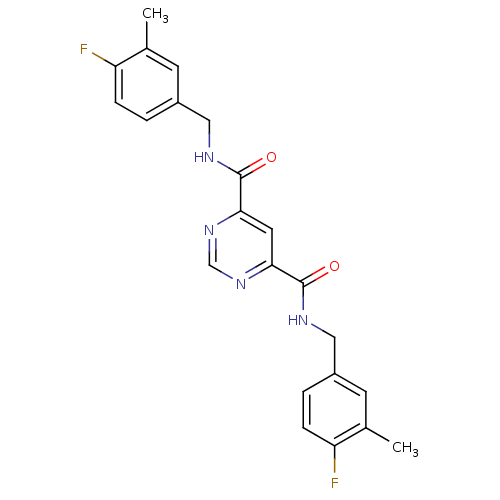
(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma Deutschland GmbH
| Assay Description
MMP-13 was assayed its proteolytic activity using a quenched fluorogenic substrate. The substrate hydrolysis was monitored by recording the increase ... |
Chem Biol 12: 181-9 (2005)
Article DOI: 10.1016/j.chembiol.2004.11.014
BindingDB Entry DOI: 10.7270/Q2WD3XTW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida Atlantic University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP13 expressed in Escherichia coli |
Bioorg Med Chem 17: 990-1005 (2009)
Article DOI: 10.1016/j.bmc.2008.03.004
BindingDB Entry DOI: 10.7270/Q2GB24ZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad CEU San Pablo
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 (unknown origin) |
J Med Chem 57: 10205-19 (2014)
Article DOI: 10.1021/jm500505f
BindingDB Entry DOI: 10.7270/Q2NK3GMC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of full-length recombinant human MMP-13 assessed as fTHP-15 substrate hydrolysis |
J Med Chem 57: 9598-611 (2014)
Article DOI: 10.1021/jm501284e
BindingDB Entry DOI: 10.7270/Q2P55Q3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant human MMP-13 assessed as bovine type-2 collagen hydrolysis after 18 hrs by ELISA |
J Med Chem 57: 9598-611 (2014)
Article DOI: 10.1021/jm501284e
BindingDB Entry DOI: 10.7270/Q2P55Q3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) using tacrin substrate |
J Med Chem 57: 9598-611 (2014)
Article DOI: 10.1021/jm501284e
BindingDB Entry DOI: 10.7270/Q2P55Q3G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 (unknown origin) using bupropion substrate |
J Med Chem 57: 9598-611 (2014)
Article DOI: 10.1021/jm501284e
BindingDB Entry DOI: 10.7270/Q2P55Q3G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) using amodiaquine substrate |
J Med Chem 57: 9598-611 (2014)
Article DOI: 10.1021/jm501284e
BindingDB Entry DOI: 10.7270/Q2P55Q3G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) using diclofenac substrate |
J Med Chem 57: 9598-611 (2014)
Article DOI: 10.1021/jm501284e
BindingDB Entry DOI: 10.7270/Q2P55Q3G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) using (S)-mephentoin substrate |
J Med Chem 57: 9598-611 (2014)
Article DOI: 10.1021/jm501284e
BindingDB Entry DOI: 10.7270/Q2P55Q3G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) using dextromethophan substrate |
J Med Chem 57: 9598-611 (2014)
Article DOI: 10.1021/jm501284e
BindingDB Entry DOI: 10.7270/Q2P55Q3G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using midazolam substrate |
J Med Chem 57: 9598-611 (2014)
Article DOI: 10.1021/jm501284e
BindingDB Entry DOI: 10.7270/Q2P55Q3G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using testosterone substrate |
J Med Chem 57: 9598-611 (2014)
Article DOI: 10.1021/jm501284e
BindingDB Entry DOI: 10.7270/Q2P55Q3G |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 19: 47-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.038
BindingDB Entry DOI: 10.7270/Q2VT1RZP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 (unknown origin) using MCA-Arg-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-Glu-Arg-NH2 as substrate preincubated for 15 mins followed by substrat... |
J Med Chem 59: 313-27 (2016)
BindingDB Entry DOI: 10.7270/Q20G3N0F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 (unknown origin) using acetyl-Pro-Leu-Gly-[2-mercapto-4-methylpentanoyl]-Leu-Gly-O-ethyl ester as substrate by microplate reader ... |
J Med Chem 59: 313-27 (2016)
BindingDB Entry DOI: 10.7270/Q20G3N0F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM16596

(4-N,6-N-bis[(4-fluoro-3-methylphenyl)methyl]pyrimi...)Show SMILES Cc1cc(CNC(=O)c2cc(ncn2)C(=O)NCc2ccc(F)c(C)c2)ccc1F Show InChI InChI=1S/C22H20F2N4O2/c1-13-7-15(3-5-17(13)23)10-25-21(29)19-9-20(28-12-27-19)22(30)26-11-16-4-6-18(24)14(2)8-16/h3-9,12H,10-11H2,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | 6.8 | 25 |
Commissariat á l'Energie Atomique
| Assay Description
Enzyme assay using human matrix metalloproteases or ADAMTS. |
J Biol Chem 287: 26647-56 (2012)
Article DOI: 10.1074/jbc.M112.380782
BindingDB Entry DOI: 10.7270/Q2H993SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data