

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM166344 US9067949, 201
SMILES: Cc1cc(cc2c3CNCCc3oc12)S(=O)(=O)c1cccc(c1)C(F)(F)F
InChI Key: InChIKey=NNXSQSVMNHMDQS-UHFFFAOYSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166344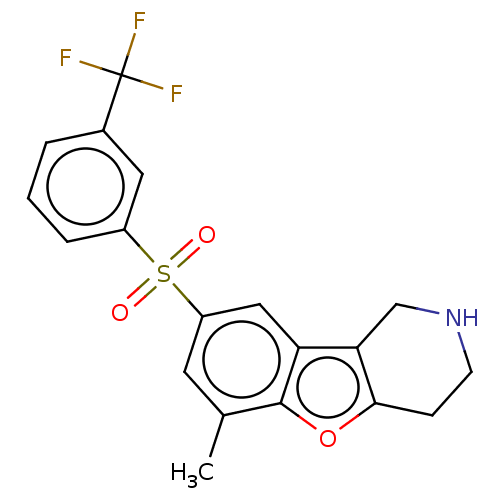 (US9067949, 201) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.220 | -13.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||