

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM171332 US9085555, 762
SMILES: NC(=O)n1cc(NC(=O)N2[C@@H]3C[C@@H]3C[C@H]2C(=O)Nc2cccc(Br)n2)c2ccccc12
InChI Key: InChIKey=NRMJBMPLENRGCS-LYRGGWFBSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complement factor D (Homo sapiens (Human)) | BDBM171332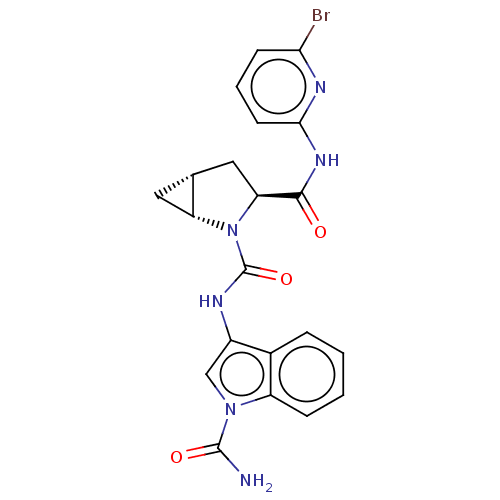 (US9085555, 762) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG US Patent | Assay Description Recombinant human factor D (expressed in E. coli and purified using standard methods) at 10 nM concentration is incubated with test compound at vario... | US Patent US9085555 (2015) BindingDB Entry DOI: 10.7270/Q2RN36MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171332 (US9085555, 762) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171332 (US9085555, 762) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM171332 (US9085555, 762) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using diclofenac as substrate | J Med Chem 60: 5717-5735 (2017) Article DOI: 10.1021/acs.jmedchem.7b00425 BindingDB Entry DOI: 10.7270/Q2TB195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||