

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM17278 3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-methylpyridin-2-yl]oxy}benzene-1-carboximidamide::CHEMBL49636::Pyridine template, III::ZK-805623
SMILES: Cc1c(F)c(Oc2cccc(c2)C(N)=N)nc(Oc2cccc(c2)C(N)=N)c1F
InChI Key: InChIKey=ZXIHYCYAQUQHSG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM17278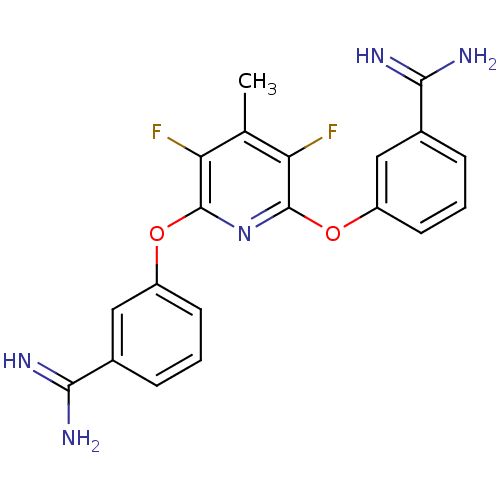 (3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17278 (3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Compound was tested for inhibition activity against Coagulation factor X | J Med Chem 42: 1749-56 (1999) Article DOI: 10.1021/jm980667k BindingDB Entry DOI: 10.7270/Q2FX78NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17278 (3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated against Coagulation factor X | Bioorg Med Chem Lett 8: 1877-82 (1999) BindingDB Entry DOI: 10.7270/Q2222SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17278 (3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory constant against human coagulation factor Xa (fXa) | J Med Chem 45: 2484-93 (2002) BindingDB Entry DOI: 10.7270/Q24J0FTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17278 (3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 14 | -10.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999) Article DOI: 10.1107/s0907444999007350 BindingDB Entry DOI: 10.7270/Q2H1308B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin-1 (Homo sapiens (Human)) | BDBM17278 (3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory constant against bovine trypsin for general specificity against serine proteases | J Med Chem 45: 2484-93 (2002) BindingDB Entry DOI: 10.7270/Q24J0FTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Bos taurus (bovine)) | BDBM17278 (3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Compound was tested for inhibition activity against bovine trypsin. | J Med Chem 42: 1749-56 (1999) Article DOI: 10.1021/jm980667k BindingDB Entry DOI: 10.7270/Q2FX78NF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypsin (Bos taurus (bovine)) | BDBM17278 (3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against bovine trypsin. | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypsin (Bos taurus (bovine)) | BDBM17278 (3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 870 | -8.18 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999) Article DOI: 10.1107/s0907444999007350 BindingDB Entry DOI: 10.7270/Q2H1308B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17278 (3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human thrombin | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17278 (3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory constant against human coagulation factor II (thrombin) for selectivity within coagulation cascade | J Med Chem 45: 2484-93 (2002) BindingDB Entry DOI: 10.7270/Q24J0FTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17278 (3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Compound was tested for inhibition activity against human thrombin (FIIa) | J Med Chem 42: 1749-56 (1999) Article DOI: 10.1021/jm980667k BindingDB Entry DOI: 10.7270/Q2FX78NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||