

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM1788 2-Amino-6-arylthiobenzonitrile deriv. 3h::2-amino-6-[(2-chlorobenzene)sulfonyl]benzonitrile
SMILES: Nc1cccc(c1C#N)S(=O)(=O)c1ccccc1Cl
InChI Key: InChIKey=NXUSIYJXPBRFJT-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Reverse Transcriptase (Human immunodeficiency virus type 1) | BDBM1788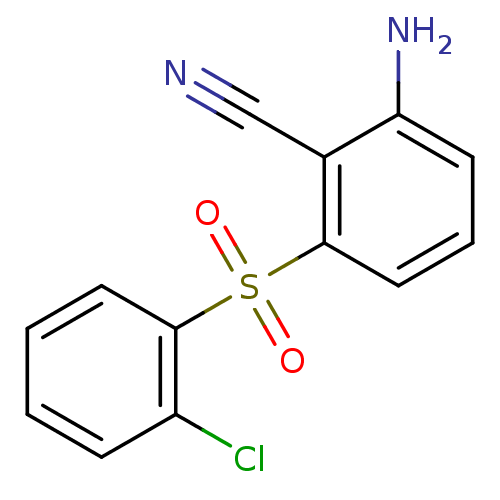 (2-Amino-6-arylthiobenzonitrile deriv. 3h | 2-amino...) | PDB MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 1866-82 (2001) Article DOI: 10.1021/jm0004906 BindingDB Entry DOI: 10.7270/Q2FJ2F09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1788 (2-Amino-6-arylthiobenzonitrile deriv. 3h | 2-amino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV-1 reverse transcriptase | Citation and Details Article DOI: 10.1007/s00044-013-0765-3 BindingDB Entry DOI: 10.7270/Q2W66PQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1788 (2-Amino-6-arylthiobenzonitrile deriv. 3h | 2-amino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 44: 2158-71 (2009) Article DOI: 10.1016/j.ejmech.2008.10.021 BindingDB Entry DOI: 10.7270/Q21R6T9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM1788 (2-Amino-6-arylthiobenzonitrile deriv. 3h | 2-amino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia Curated by ChEMBL | Assay Description Inhibitory concentration against Reverse transcriptase | J Med Chem 48: 3756-67 (2005) Article DOI: 10.1021/jm049162m BindingDB Entry DOI: 10.7270/Q2XS5WQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||