

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM17943 Renin nonpeptide inhibitor, 3::methyl (3S)-1-[(5S,6S,8R)-5-amino-8-(butylcarbamoyl)-6-hydroxy-3,3,8-trimethyloctanoyl]-1,2,3,4-tetrahydroquinoline-3-carboxylate
SMILES: CCCCNC(=O)[C@H](C)C[C@H](O)[C@@H](N)CC(C)(C)CC(=O)N1C[C@H](Cc2ccccc12)C(=O)OC
InChI Key: InChIKey=VROPGBJWKDHPPG-ANZJIFDASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM17943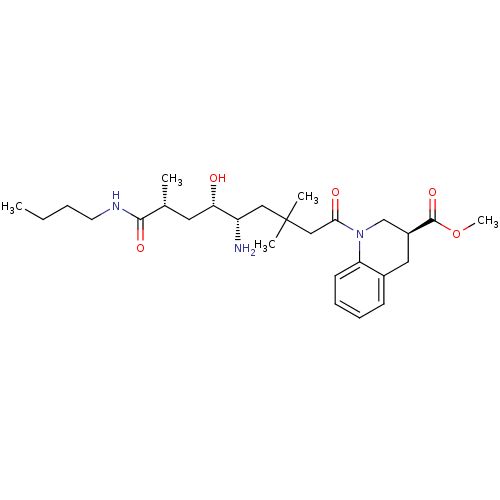 (Renin nonpeptide inhibitor, 3 | methyl (3S)-1-[(5S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified human recombinant renin were determined by its cleavage of substrate angiotensinogen. The angiotensi... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17943 (Renin nonpeptide inhibitor, 3 | methyl (3S)-1-[(5S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU) Curated by ChEMBL | Assay Description Inhibition of human renin | Eur J Med Chem 46: 2469-76 (2011) Article DOI: 10.1016/j.ejmech.2011.03.035 BindingDB Entry DOI: 10.7270/Q2GM87NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pepsin A (Porcine) | BDBM17943 (Renin nonpeptide inhibitor, 3 | methyl (3S)-1-[(5S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 2.0 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified porcine pepsin were determined by its cleavage of substrate hemoglobin. After incubation, proteins w... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||