

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM179801 3-((2,6-dichloro-3-fluorophenyl)thio)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)-1H-pyrazolo[3,4-b]pyridine (9)
SMILES: Fc1ccc(Cl)c(Sc2n[nH]c3ncc(cc23)-c2cnn(c2)C2CCNCC2)c1Cl
InChI Key: InChIKey=FCLVBOUURHAHBG-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine kinase receptor c-Met (c-Met) (Homo sapiens (Human)) | BDBM179801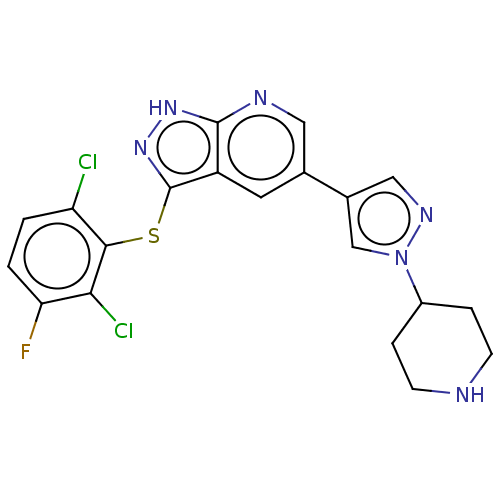 (3-((2,6-dichloro-3-fluorophenyl)thio)-5-(1-(piperi...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22.8 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Tianjin University of Science and Technology | Assay Description The c-Met kinase activity of five target compounds and three positive compounds were evaluated using standard Z-LYTE Assays (fluorescence resonance e... | Bioorg Chem 65: 146-58 (2016) Article DOI: 10.1016/j.bioorg.2016.02.009 BindingDB Entry DOI: 10.7270/Q2VT1QVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (L1196M) (Homo sapiens (Human)) | BDBM179801 (3-((2,6-dichloro-3-fluorophenyl)thio)-5-(1-(piperi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 578 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Tianjin University of Science and Technology | Assay Description The ALK kinase activity of five target compounds and three positive compounds were evaluated using standard homogeneous time-resolved fluorescence (H... | Bioorg Chem 65: 146-58 (2016) Article DOI: 10.1016/j.bioorg.2016.02.009 BindingDB Entry DOI: 10.7270/Q2VT1QVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anaplastic lymphoma kinase C1156Y (ALK C1156Y) (Homo sapiens (Human)) | BDBM179801 (3-((2,6-dichloro-3-fluorophenyl)thio)-5-(1-(piperi...) | PDB MMDB KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 651 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Tianjin University of Science and Technology | Assay Description The ALK kinase activity of five target compounds and three positive compounds were evaluated using standard homogeneous time-resolved fluorescence (H... | Bioorg Chem 65: 146-58 (2016) Article DOI: 10.1016/j.bioorg.2016.02.009 BindingDB Entry DOI: 10.7270/Q2VT1QVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM179801 (3-((2,6-dichloro-3-fluorophenyl)thio)-5-(1-(piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Tianjin University of Science and Technology | Assay Description The ALK kinase activity of five target compounds and three positive compounds were evaluated using standard homogeneous time-resolved fluorescence (H... | Bioorg Chem 65: 146-58 (2016) Article DOI: 10.1016/j.bioorg.2016.02.009 BindingDB Entry DOI: 10.7270/Q2VT1QVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||