

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM18012 trans,trans-4-arylpiperidine-based compound, 1
SMILES: COC[C@@H](O)CO[C@@H]1CNC[C@H](OCc2cc(OC)c3ccccc3c2)[C@H]1c1ccc(OCCCOCc2ccccc2OC)cc1
InChI Key: InChIKey=NSAUOOQLSGAVDH-YYZIUVEKSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM18012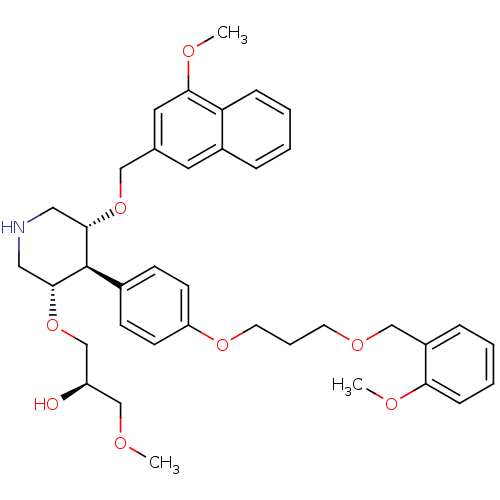 (trans,trans-4-arylpiperidine-based compound, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem Lett 17: 3575-80 (2007) Article DOI: 10.1016/j.bmcl.2007.04.052 BindingDB Entry DOI: 10.7270/Q2B56H0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18012 (trans,trans-4-arylpiperidine-based compound, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of plasma renin (unknown origin) | J Med Chem 56: 2196-206 (2013) Article DOI: 10.1021/jm301706j BindingDB Entry DOI: 10.7270/Q25X2B8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18012 (trans,trans-4-arylpiperidine-based compound, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18012 (trans,trans-4-arylpiperidine-based compound, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of renin in plasma | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||