 Found 9 hits for monomerid = 18046
Found 9 hits for monomerid = 18046 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Escherichia coli) | BDBM18046
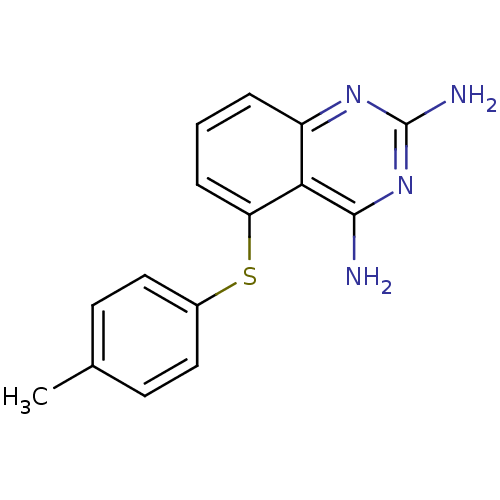
(5-((4-methylphenyl)thio)-quinazoline 2,4-diamine, ...)Show InChI InChI=1S/C15H14N4S/c1-9-5-7-10(8-6-9)20-12-4-2-3-11-13(12)14(16)19-15(17)18-11/h2-8H,1H3,(H4,16,17,18,19) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Dihydrofolate reductase in presence of 30 uM Dihydrofolate reductase |
Bioorg Med Chem Lett 13: 2493-6 (2003)
BindingDB Entry DOI: 10.7270/Q2CN74G5 |
More data for this
Ligand-Target Pair | |
Dihydrofolate Reductase (DHFR)
(Escherichia coli) | BDBM18046

(5-((4-methylphenyl)thio)-quinazoline 2,4-diamine, ...)Show InChI InChI=1S/C15H14N4S/c1-9-5-7-10(8-6-9)20-12-4-2-3-11-13(12)14(16)19-15(17)18-11/h2-8H,1H3,(H4,16,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
| Assay Description
Inhibition assay using Esherichia coli dihydrofolate reductase (DHFR). |
Chem Biol 11: 1423-30 (2004)
Article DOI: 10.1016/j.chembiol.2004.08.014
BindingDB Entry DOI: 10.7270/Q2639N6G |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18046

(5-((4-methylphenyl)thio)-quinazoline 2,4-diamine, ...)Show InChI InChI=1S/C15H14N4S/c1-9-5-7-10(8-6-9)20-12-4-2-3-11-13(12)14(16)19-15(17)18-11/h2-8H,1H3,(H4,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Research Institute, Hospital for Sick Children
| Assay Description
Beta-glucocerebrosidase (GCase) activity was measured by release of 4-methylumbelliferyl fluorophore from MUClc. |
Chembiochem 9: 2650-62 (2008)
Article DOI: 10.1002/cbic.200800304
BindingDB Entry DOI: 10.7270/Q2JQ0ZJK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18046

(5-((4-methylphenyl)thio)-quinazoline 2,4-diamine, ...)Show InChI InChI=1S/C15H14N4S/c1-9-5-7-10(8-6-9)20-12-4-2-3-11-13(12)14(16)19-15(17)18-11/h2-8H,1H3,(H4,16,17,18,19) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Dihydrofolate reductase in presence of 100 uM Dihydrofolate reductase |
Bioorg Med Chem Lett 13: 2493-6 (2003)
BindingDB Entry DOI: 10.7270/Q2CN74G5 |
More data for this
Ligand-Target Pair | |
Dihydrofolate Reductase (DHFR)
(Candida albicans) | BDBM18046

(5-((4-methylphenyl)thio)-quinazoline 2,4-diamine, ...)Show InChI InChI=1S/C15H14N4S/c1-9-5-7-10(8-6-9)20-12-4-2-3-11-13(12)14(16)19-15(17)18-11/h2-8H,1H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company
Curated by ChEMBL
| Assay Description
Inhibition of dihydrofolate reductase in Candida albicans (in vitro). |
J Med Chem 38: 3608-16 (1995)
BindingDB Entry DOI: 10.7270/Q2V69KS6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18046

(5-((4-methylphenyl)thio)-quinazoline 2,4-diamine, ...)Show InChI InChI=1S/C15H14N4S/c1-9-5-7-10(8-6-9)20-12-4-2-3-11-13(12)14(16)19-15(17)18-11/h2-8H,1H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human dihydrofolate reductase |
J Med Chem 38: 3608-16 (1995)
BindingDB Entry DOI: 10.7270/Q2V69KS6 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18046

(5-((4-methylphenyl)thio)-quinazoline 2,4-diamine, ...)Show InChI InChI=1S/C15H14N4S/c1-9-5-7-10(8-6-9)20-12-4-2-3-11-13(12)14(16)19-15(17)18-11/h2-8H,1H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | 7.0 | 30 |
GSK
| Assay Description
IC50 is the concentration of inhibitor that decreases the velocity of the standard assay by 50%. The enzyme, NADPH, and varying concentrations of inh... |
J Med Chem 44: 2928-32 (2001)
Article DOI: 10.1021/jm0101444
BindingDB Entry DOI: 10.7270/Q2XW4H2K |
More data for this
Ligand-Target Pair | |
Dihydrofolate Reductase (DHFR)
(Candida albicans) | BDBM18046

(5-((4-methylphenyl)thio)-quinazoline 2,4-diamine, ...)Show InChI InChI=1S/C15H14N4S/c1-9-5-7-10(8-6-9)20-12-4-2-3-11-13(12)14(16)19-15(17)18-11/h2-8H,1H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 6.4 | 30 |
GSK
| Assay Description
IC50 is the concentration of inhibitor that decreases the velocity of the standard assay by 50%. The enzyme, NADPH, and varying concentrations of inh... |
J Med Chem 44: 2928-32 (2001)
Article DOI: 10.1021/jm0101444
BindingDB Entry DOI: 10.7270/Q2XW4H2K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Escherichia coli) | BDBM18046

(5-((4-methylphenyl)thio)-quinazoline 2,4-diamine, ...)Show InChI InChI=1S/C15H14N4S/c1-9-5-7-10(8-6-9)20-12-4-2-3-11-13(12)14(16)19-15(17)18-11/h2-8H,1H3,(H4,16,17,18,19) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Dihydrofolate reductase in presence of 30 uM Dihydrofolate reductase |
Bioorg Med Chem Lett 13: 2493-6 (2003)
BindingDB Entry DOI: 10.7270/Q2CN74G5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data