

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM18047 5-[(4-TERT-BUTYLPHENYL)SULFANYL]-2,4-QUINAZOLINEDIAMINE::5-[(4-tert-butylphenyl)sulfanyl]quinazoline-2,4-diamine::GW995
SMILES: CC(C)(C)c1ccc(Sc2cccc3nc(N)nc(N)c23)cc1
InChI Key: InChIKey=MNYDVPDMLAJJPB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate Reductase (DHFR) (Candida albicans) | BDBM18047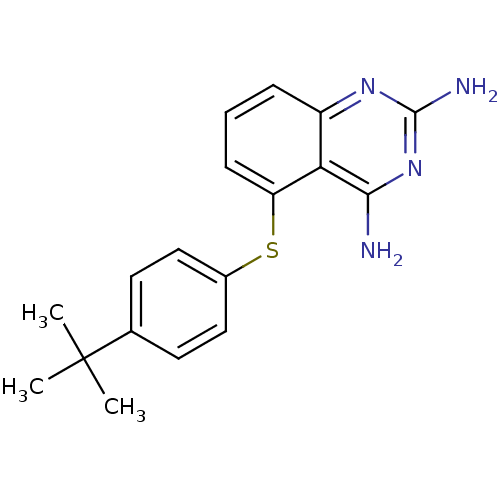 (5-[(4-TERT-BUTYLPHENYL)SULFANYL]-2,4-QUINAZOLINEDI...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 6.4 | 30 |
GSK | Assay Description IC50 is the concentration of inhibitor that decreases the velocity of the standard assay by 50%. The enzyme, NADPH, and varying concentrations of inh... | J Med Chem 44: 2928-32 (2001) Article DOI: 10.1021/jm0101444 BindingDB Entry DOI: 10.7270/Q2XW4H2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate Reductase (DHFR) (Candida albicans) | BDBM18047 (5-[(4-TERT-BUTYLPHENYL)SULFANYL]-2,4-QUINAZOLINEDI...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description Inhibition of dihydrofolate reductase in Candida albicans (in vitro). | J Med Chem 38: 3608-16 (1995) BindingDB Entry DOI: 10.7270/Q2V69KS6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18047 (5-[(4-TERT-BUTYLPHENYL)SULFANYL]-2,4-QUINAZOLINEDI...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description In vitro inhibition of human dihydrofolate reductase | J Med Chem 38: 3608-16 (1995) BindingDB Entry DOI: 10.7270/Q2V69KS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18047 (5-[(4-TERT-BUTYLPHENYL)SULFANYL]-2,4-QUINAZOLINEDI...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 30 |
GSK | Assay Description IC50 is the concentration of inhibitor that decreases the velocity of the standard assay by 50%. The enzyme, NADPH, and varying concentrations of inh... | J Med Chem 44: 2928-32 (2001) Article DOI: 10.1021/jm0101444 BindingDB Entry DOI: 10.7270/Q2XW4H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||