

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM18136 ADP, alpha beta-me::AMPCPP::[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)methyl]phosphonic acid::alpha, beta-methyleneadenosine 5 -diphosphate::cid_92199
SMILES: Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O
InChI Key: InChIKey=OLCWZBFDIYXLAA-IOSLPCCCSA-N
PDB links: 4 PDB IDs match this monomer. 46 PDB IDs contain this monomer as substructures. 46 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM18136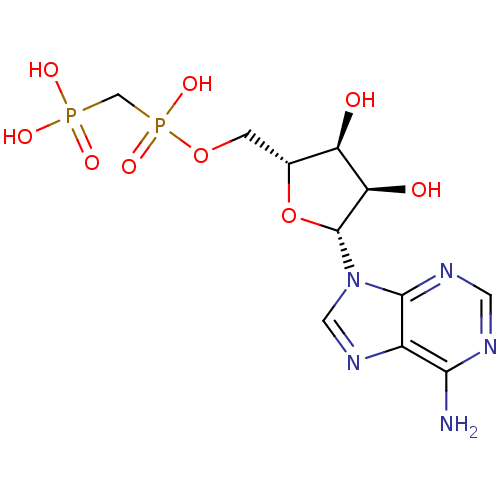 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of purified recombinant soluble human CD73 expressed in Sf9 cells [3H]AMP as substrate incubated for 25 mins by scintillation counting met... | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM18136 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arcus Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human CD73 | J Med Chem 63: 3935-3955 (2020) Article DOI: 10.1021/acs.jmedchem.9b01713 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecto-5'-nucleotidase (e5'NT) (Rattus norvegicus (Rat)) | BDBM18136 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecto-5'-nucleotidase (e5'NT) (Rattus norvegicus (Rat)) | BDBM18136 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of purified recombinant soluble rat CD73 expressed in Sf9 cells [3H]AMP as substrate incubated for 25 mins by scintillation counting metho... | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM18136 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of native CD73 in human MDA-MB-231 cell membrane preparations [3H]AMP as substrate incubated for 25 mins by scintillation counting method | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM18136 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 407-16 (2004) Article DOI: 10.1124/jpet.103.064907 BindingDB Entry DOI: 10.7270/Q2SQ8XZR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 2 (Homo sapiens (Human)) | BDBM18136 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 407-16 (2004) Article DOI: 10.1124/jpet.103.064907 BindingDB Entry DOI: 10.7270/Q2SQ8XZR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| tRNA synthetase (GlyRS) (Bombyx mori) | BDBM18136 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.00E+4 | -5.76 | 6.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Medical College of Ohio | Assay Description Aminoacyl-tRNA synthetase assays were measuring the incorporation of [14C] amino acid into tRNA. | Biochemistry 42: 5333-40 (2003) Article DOI: 10.1021/bi030031h BindingDB Entry DOI: 10.7270/Q2HQ3X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM18136 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cells assessed as reduction in conversion of AMP to adenosine incubated for 30 mins by malachite green reagent... | Eur J Med Chem 157: 1051-1055 (2018) Article DOI: 10.1016/j.ejmech.2018.08.035 BindingDB Entry DOI: 10.7270/Q2QJ7M16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM18136 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of CD73 in human NCI-H292 cells assessed as reduction in conversion of AMP to adenosine incubated for 30 mins by malachite green reagent b... | Eur J Med Chem 157: 1051-1055 (2018) Article DOI: 10.1016/j.ejmech.2018.08.035 BindingDB Entry DOI: 10.7270/Q2QJ7M16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase C-beta-3 (Homo sapiens (Human)) | BDBM18136 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | 1.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute Ass... | PubChem Bioassay (2014) BindingDB Entry DOI: 10.7270/Q2S46QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase C, gamma 1 (Homo sapiens (Human)) | BDBM18136 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute Ass... | PubChem Bioassay (2014) BindingDB Entry DOI: 10.7270/Q2NC5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM18136 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of recombinant CD73 (27 to 549 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells assessed as reduction in conv... | Eur J Med Chem 157: 1051-1055 (2018) Article DOI: 10.1016/j.ejmech.2018.08.035 BindingDB Entry DOI: 10.7270/Q2QJ7M16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||