

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM18162 (1R,6R,7S)-4-(4-nitronaphthalen-1-yl)-2,4-diazatricyclo[5.2.1.0^{2,6}]decane-3,5-dione::CHEMBL185880::N-aryl-bicyclic hydantoin, 4a
SMILES: Oc1c2[C@H]3CC[C@H](C3)n2c(=O)n1-c1ccc([N+]([O-])=O)c2ccccc12
InChI Key: InChIKey=MWACATADSGPYNM-WDEREUQCSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Homo sapiens (Human)) | BDBM18162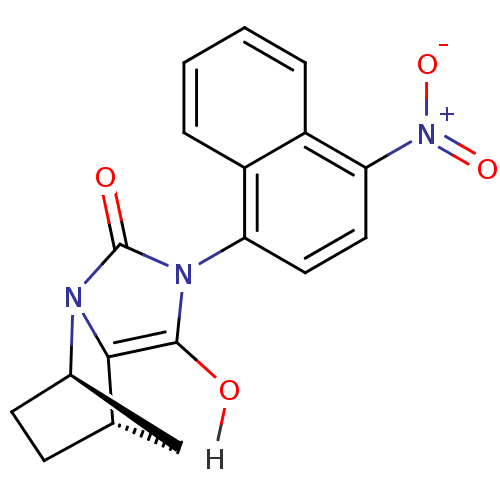 ((1R,6R,7S)-4-(4-nitronaphthalen-1-yl)-2,4-diazatri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-DHT from androgen receptor of human MDA-453 cells | Bioorg Med Chem Lett 14: 6107-11 (2004) Article DOI: 10.1016/j.bmcl.2004.09.049 BindingDB Entry DOI: 10.7270/Q2T1533W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18162 ((1R,6R,7S)-4-(4-nitronaphthalen-1-yl)-2,4-diazatri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | -12.1 | n/a | n/a | 385 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 49: 7596-9 (2006) Article DOI: 10.1021/jm061101w BindingDB Entry DOI: 10.7270/Q2862DQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18162 ((1R,6R,7S)-4-(4-nitronaphthalen-1-yl)-2,4-diazatri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonistic activity against androgen receptor of MDA-453 cells | Bioorg Med Chem Lett 14: 6107-11 (2004) Article DOI: 10.1016/j.bmcl.2004.09.049 BindingDB Entry DOI: 10.7270/Q2T1533W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18162 ((1R,6R,7S)-4-(4-nitronaphthalen-1-yl)-2,4-diazatri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonistic activity against mutant androgen receptor of LNCap cells | Bioorg Med Chem Lett 14: 6107-11 (2004) Article DOI: 10.1016/j.bmcl.2004.09.049 BindingDB Entry DOI: 10.7270/Q2T1533W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||