

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM184561 US9150581, RTI-7527-(+/-)-102::US9150581, RTI-7527-(-)-102
SMILES: Fc1ncc(cc1-c1ccc(cc1)N(=O)=O)C1CC2CCC1N2
InChI Key: InChIKey=VYBKMQYOOUBJRG-UHFFFAOYSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM184561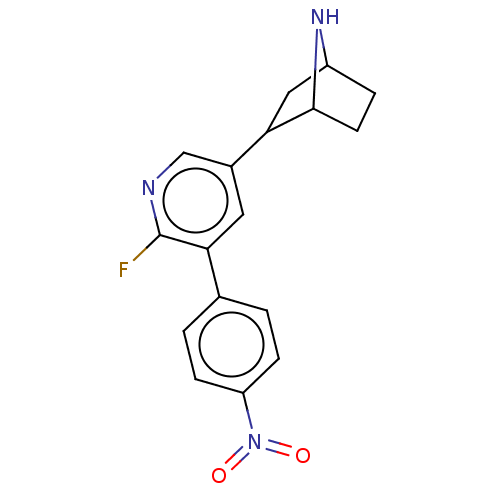 (US9150581, RTI-7527-(+/-)-102 | US9150581, RTI-752...) | PDB GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM184561 (US9150581, RTI-7527-(+/-)-102 | US9150581, RTI-752...) | PDB GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM184561 (US9150581, RTI-7527-(+/-)-102 | US9150581, RTI-752...) | PDB GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM184561 (US9150581, RTI-7527-(+/-)-102 | US9150581, RTI-752...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description Compounds (10 mM) were also evaluated for inhibition of binding to a7 nAChR using [125I]iodoMLA as previously reported in Carroll et al. The binding ... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||