 Found 6 hits for monomerid = 185147
Found 6 hits for monomerid = 185147 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ferrochelatase, mitochondrial [R115L]
(Homo sapiens (Human)) | BDBM185147
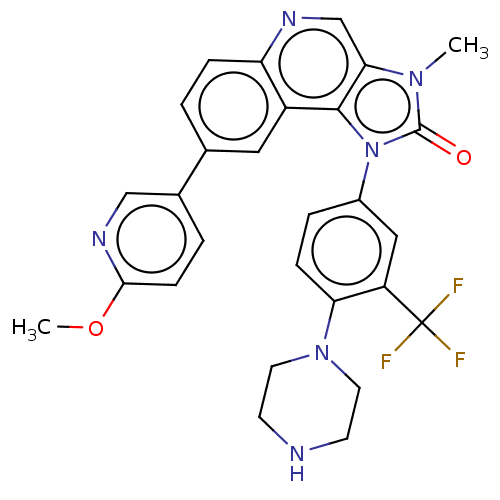
((Z)-but-2-enedioic acid;8-(6-methoxypyridin-3-yl)-...)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(-c4ccc(N5CCNCC5)c(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C28H25F3N6O2/c1-35-24-16-33-22-6-3-17(18-4-8-25(39-2)34-15-18)13-20(22)26(24)37(27(35)38)19-5-7-23(21(14-19)28(29,30)31)36-11-9-32-10-12-36/h3-8,13-16,32H,9-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... |
ACS Chem Biol 11: 1245-54 (2016)
Article DOI: 10.1021/acschembio.5b01063
BindingDB Entry DOI: 10.7270/Q2FB51R8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM185147

((Z)-but-2-enedioic acid;8-(6-methoxypyridin-3-yl)-...)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(-c4ccc(N5CCNCC5)c(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C28H25F3N6O2/c1-35-24-16-33-22-6-3-17(18-4-8-25(39-2)34-15-18)13-20(22)26(24)37(27(35)38)19-5-7-23(21(14-19)28(29,30)31)36-11-9-32-10-12-36/h3-8,13-16,32H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 41.9 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ZUANZHU PHARMA CO., LTD.
US Patent
| Assay Description
1. Preparation of test reagents{circle around (1)} 1x kinase buffer (50 mM HEPES, pH 7.5, 3 mM MgCl2, 1 mM EGTA, 100 mM NaCl, 0.03% CHAPS, 2 mM DTT);... |
US Patent US9284315 (2016)
BindingDB Entry DOI: 10.7270/Q2NC6011 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM185147

((Z)-but-2-enedioic acid;8-(6-methoxypyridin-3-yl)-...)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(-c4ccc(N5CCNCC5)c(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C28H25F3N6O2/c1-35-24-16-33-22-6-3-17(18-4-8-25(39-2)34-15-18)13-20(22)26(24)37(27(35)38)19-5-7-23(21(14-19)28(29,30)31)36-11-9-32-10-12-36/h3-8,13-16,32H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
Bioorg Med Chem 26: 4537-4543 (2018)
Article DOI: 10.1016/j.bmc.2018.07.047
BindingDB Entry DOI: 10.7270/Q20C4ZDW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM185147

((Z)-but-2-enedioic acid;8-(6-methoxypyridin-3-yl)-...)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(-c4ccc(N5CCNCC5)c(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C28H25F3N6O2/c1-35-24-16-33-22-6-3-17(18-4-8-25(39-2)34-15-18)13-20(22)26(24)37(27(35)38)19-5-7-23(21(14-19)28(29,30)31)36-11-9-32-10-12-36/h3-8,13-16,32H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem 26: 4537-4543 (2018)
Article DOI: 10.1016/j.bmc.2018.07.047
BindingDB Entry DOI: 10.7270/Q20C4ZDW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM185147

((Z)-but-2-enedioic acid;8-(6-methoxypyridin-3-yl)-...)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(-c4ccc(N5CCNCC5)c(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C28H25F3N6O2/c1-35-24-16-33-22-6-3-17(18-4-8-25(39-2)34-15-18)13-20(22)26(24)37(27(35)38)19-5-7-23(21(14-19)28(29,30)31)36-11-9-32-10-12-36/h3-8,13-16,32H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
Bioorg Med Chem 26: 4537-4543 (2018)
Article DOI: 10.1016/j.bmc.2018.07.047
BindingDB Entry DOI: 10.7270/Q20C4ZDW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM185147

((Z)-but-2-enedioic acid;8-(6-methoxypyridin-3-yl)-...)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(-c4ccc(N5CCNCC5)c(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C28H25F3N6O2/c1-35-24-16-33-22-6-3-17(18-4-8-25(39-2)34-15-18)13-20(22)26(24)37(27(35)38)19-5-7-23(21(14-19)28(29,30)31)36-11-9-32-10-12-36/h3-8,13-16,32H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ZUANZHU PHARMA CO., LTD.
US Patent
| Assay Description
1. Preparation of test reagents {circle around (1)} 1x kinase buffer (50 mM HEPES, pH 7.5, 10 mM MgCl2, 1 mM EGTA, 3 mM MnCl, 0.01% Tween-20, 2 mM DT... |
US Patent US9284315 (2016)
BindingDB Entry DOI: 10.7270/Q2NC6011 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data