

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM185685 UNC10107966::US9156822, 15
SMILES: Clc1cccc(N2CCCN(CCCOc3ccc4ccc(=O)[nH]c4c3)CC2)c1Cl
InChI Key: InChIKey=WXHPNHJKGIFCOC-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185685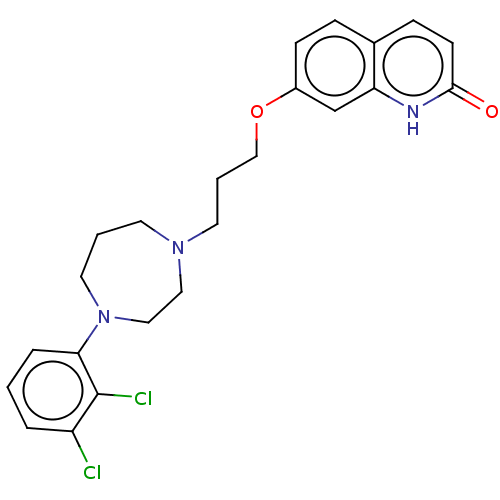 (UNC10107966 | US9156822, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185685 (UNC10107966 | US9156822, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Recruitment of β-arrestin to agonist-stimulated D2 receptors was performed using a previously described Tango-type assay (Barnea et al., Proc. N... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185685 (UNC10107966 | US9156822, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 13 | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description HEK293T cells co-expressing the cAMP biosensor GloSensor-22F (Promega) and hD2 receptors were seeded (10,000 cells/20 ul/well) into white, clear-bott... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||