

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM18787 5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]pyrimidine-2,4-diamine::CHEMBL22403::P40
SMILES: Nc1nc(N)c(c(CCOCCCOc2ccccc2)n1)-c1cccc(Cl)c1
InChI Key: InChIKey=CRGQDUYGPMHVEY-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787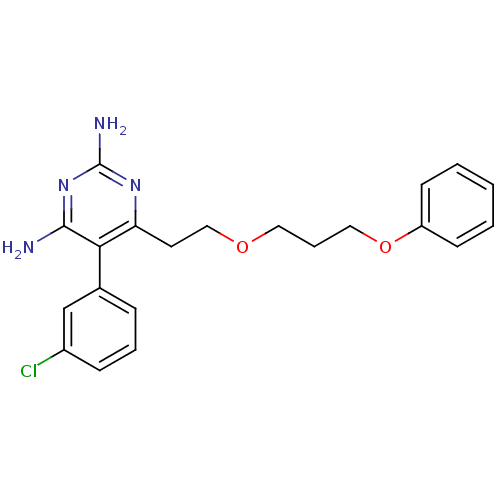 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description In vitro anti-plasmodial activity against Plasmodium falciparum with wild type dihydrofolate reductase (TM4/8.2). | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 0.630 | -12.5 | 2.27E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description In vitro anti-plasmodial activity against Plasmodium falciparum with mutant C59R+S108N+I164L (Csl-2) dihydrofolate reductase. | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Antiplasmodial activity (IC50) against Plasmodium falciparum Clone with mutant enzyme C59R+S108N- pfDihydrofolate reductase (K1CB1) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description In vitro anti-plasmodial activity against Plasmodium falciparum with mutant N51I+C59R+S108N (W2) dihydrofolate reductase. | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description In vitro anti-plasmodial activity against Plasmodium falciparum with mutant CN51I+C59R+S108N+I164L (V1/S) dihydrofolate reductase. | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [1-238,S58R,S117N] (Plasmodium vivax (malaria parasite P. vivax)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 1.53 | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology at Thailand | Assay Description Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... | Antimicrob Agents Chemother 50: 3631-7 (2006) Article DOI: 10.1128/AAC.00448-06 BindingDB Entry DOI: 10.7270/Q2N8781P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the S108N mutant of dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Binding affinity towards mutant dihydrofolate reductase (C59R+S108N+I164L DHFR) of Plasmodium falciparum | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Binding affinity towards mutant dihydrofolate reductase (N51I+C59R+S108N+I164L DHFR) of Plasmodium falciparum | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Binding affinity towards wild-type dihydrofolate reductase of Plasmodium falciparum. | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition constant against Plasmodium falciparum dihydrofolate reductase | J Med Chem 47: 4258-67 (2004) Checked by Author Article DOI: 10.1021/jm040769c BindingDB Entry DOI: 10.7270/Q2HH6JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Binding affinity towards mutant dihydrofolate reductase (N51I+C59R+S108N DHFR) of Plasmodium falciparum | J Med Chem 47: 673-80 (2004) Article DOI: 10.1021/jm030165t BindingDB Entry DOI: 10.7270/Q2ST7P8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the C59R+S108N mutant of dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||