

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM18958 (2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-diiodophenoxy}ethyl)(ethyl)amine::CHEMBL1600::DEA::Deethylamiodarone::Desethylamiodarone::L 33520
SMILES: CCCCc1oc2ccccc2c1C(=O)c1cc(I)c(OCCNCC)c(I)c1
InChI Key: InChIKey=VXOKDLACQICQFA-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thyroid Hormone Receptor (TR-alpha) (Gallus gallus (chicken)) | BDBM18958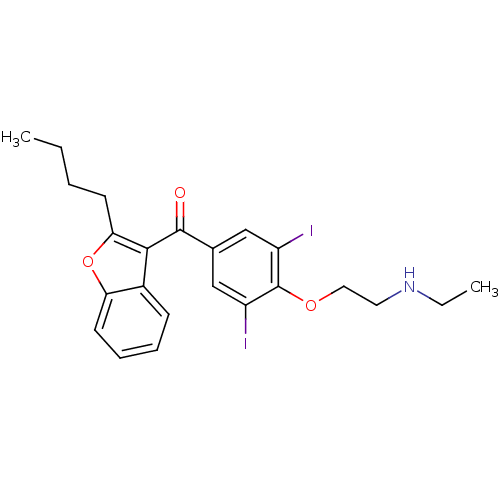 ((2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | 7.6 | 22 |
University of Amsterdam | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. | Endocrinology 137: 2807-14 (1996) Article DOI: 10.1210/endo.137.7.8770901 BindingDB Entry DOI: 10.7270/Q2ZK5DX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| thyroid beta (RAT) | BDBM18958 ((2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | 7.6 | 22 |
University of Amsterdam | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. | Endocrinology 137: 2807-14 (1996) Article DOI: 10.1210/endo.137.7.8770901 BindingDB Entry DOI: 10.7270/Q2ZK5DX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM18958 ((2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-di...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Digoxin transepithelial transport (basal to apical) (Digoxin: 0.025 uM) in MDR1-expressing LLC-PK1 cells | Eur J Pharm Sci 12: 505-13 (2001) BindingDB Entry DOI: 10.7270/Q2JH3NG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM18958 ((2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-di...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Daunorubicin transepithelial transport (basal to apical) (Daunorubicin: 0.035 uM) in MDR1-expressing LLC-PK1 cells | Eur J Pharm Sci 12: 505-13 (2001) BindingDB Entry DOI: 10.7270/Q2JH3NG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (TAAR1) (Rattus norvegicus (Rat)) | BDBM18958 ((2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-di...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
University of California at San Francisco Curated by ChEMBL | Assay Description Antagonist activity at rat TAAR1 expressed in HEK293 cells assessed as intracellular cAMP level | Bioorg Med Chem Lett 18: 5920-2 (2008) Article DOI: 10.1016/j.bmcl.2008.08.013 BindingDB Entry DOI: 10.7270/Q2HQ3ZQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||