

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM189884 US10227346, Example 11::US10426135, Example 11::US9670201, 11 4-chloro-3-cyano-N-(3-(1-isobutyrylpiperidin-4-yl)-1-methyl-4-(trifluoromethyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)benzamide::US9920054, Example 11
SMILES: CC(C)C(=O)N1CCC(CC1)c1cn(C)c2ncc(NC(=O)c3ccc(Cl)c(c3)C#N)c(c12)C(F)(F)F
InChI Key: InChIKey=UGNVAFBQCWZNTR-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM189884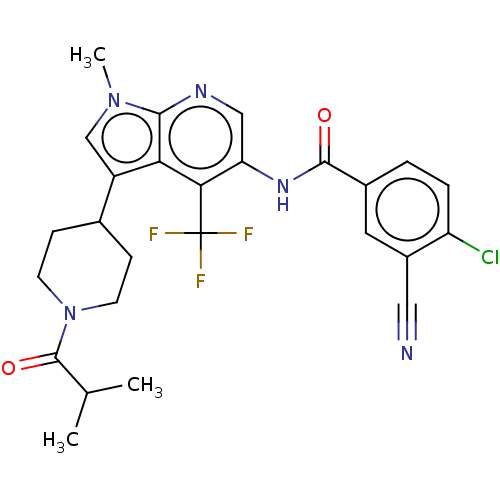 (US10227346, Example 11 | US10426135, Example 11 | ...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. US Patent | Assay Description Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl... | US Patent US9670201 (2017) BindingDB Entry DOI: 10.7270/Q28G8HWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM189884 (US10227346, Example 11 | US10426135, Example 11 | ...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | US Patent US10426135 (2019) BindingDB Entry DOI: 10.7270/Q23N25QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM189884 (US10227346, Example 11 | US10426135, Example 11 | ...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | J Med Chem 50: 4928-38 (2007) BindingDB Entry DOI: 10.7270/Q2GH9M91 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM189884 (US10227346, Example 11 | US10426135, Example 11 | ...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2SF2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||