

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM190405 US9180183, Quinidine
SMILES: COc1ccc2nccc([C@H](O)C3CC4CCN3C[C@@H]4C=C)c2c1
InChI Key: InChIKey=LOUPRKONTZGTKE-QPWZMSJCSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM190405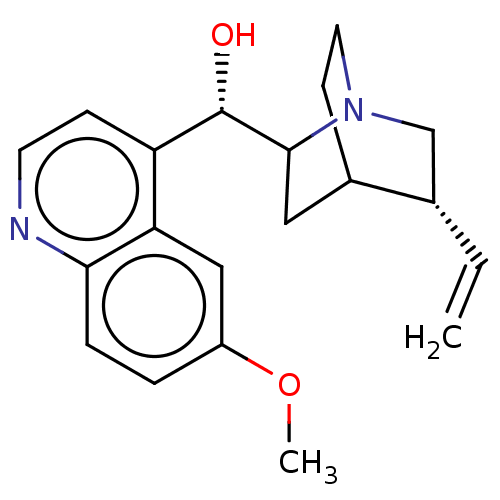 (US9180183, Quinidine) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM190405 (US9180183, Quinidine) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM190405 (US9180183, Quinidine) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Twik-RElated Potassium (K+) channel 1 (TREK1) (Homo sapiens (Human)) | BDBM190405 (US9180183, Quinidine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.41E+4 | n/a | n/a | 7.3 | 22 |
Korea Institute of Science and Technology | Assay Description The hTREK1 stable cell lines were seeded at a density of 10 000 cells/well in a 12-well plate. Whole-cell membrane currents were amplified using the ... | Chem Biol Drug Des 88: 807-819 (2016) Article DOI: 10.1111/cbdd.12810 BindingDB Entry DOI: 10.7270/Q2S181B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||