

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM190650 LOX inhibitor, MLS000545091::PT91
SMILES: CCCCCCc1ccc(Oc2ccccc2Cl)c(O)c1
InChI Key: InChIKey=ASRSXDRAEGLYDZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM190650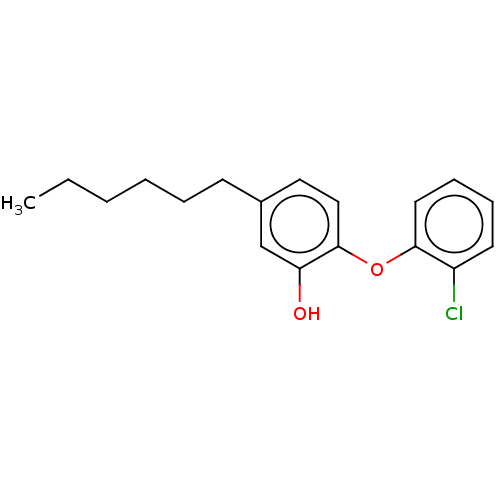 (LOX inhibitor, MLS000545091 | PT91) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Agonistic activity against progesterone receptor in alkaline phosphatase assay using human T47D breast carcinoma cell line | Eur J Med Chem 146: 318-343 (2018) Article DOI: 10.1016/j.ejmech.2018.01.047 BindingDB Entry DOI: 10.7270/Q2GF0X12 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-ACP reductase (FabI1) (Burkholderia pseudomallei) | BDBM190650 (LOX inhibitor, MLS000545091 | PT91) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 25 | -10.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University | Assay Description Slow-onset inhibition kinetics were monitored at 340 nm on a Cary 100 spectrophotometer (Varian) at 25 °C in 30 mM PIPES buffer (pH 8.0) containing 1... | Biochemistry 56: 1865-1878 (2017) Article DOI: 10.1021/acs.biochem.6b01048 BindingDB Entry DOI: 10.7270/Q2NP239F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM190650 (LOX inhibitor, MLS000545091 | PT91) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis InhA | Eur J Med Chem 146: 318-343 (2018) Article DOI: 10.1016/j.ejmech.2018.01.047 BindingDB Entry DOI: 10.7270/Q2GF0X12 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-ACP reductase T276S (T276S ypFabV) (Yersinia pestis (Enterobacteria)) | BDBM190650 (LOX inhibitor, MLS000545091 | PT91) | PDB GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg | Assay Description IC50 values for the inhibition of wildtype and mutant forms of ypFabV were performed by adding varying concentrations of inhibitor dissolved in dimet... | Biochemistry 55: 2992-3006 (2016) Article DOI: 10.1021/acs.biochem.5b01301 BindingDB Entry DOI: 10.7270/Q25D8QM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Wt ypFabV) (Yersinia pestis (Enterobacteria)) | BDBM190650 (LOX inhibitor, MLS000545091 | PT91) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg | Assay Description IC50 values for the inhibition of wildtype and mutant forms of ypFabV were performed by adding varying concentrations of inhibitor dissolved in dimet... | Biochemistry 55: 2992-3006 (2016) Article DOI: 10.1021/acs.biochem.5b01301 BindingDB Entry DOI: 10.7270/Q25D8QM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-Lipoxygenase (LoxA) (Pseudomonas aeruginosa) | BDBM190650 (LOX inhibitor, MLS000545091 | PT91) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of California, Santa Cruz | Assay Description The one-point inhibition percentages were determined for human LOX inhibitors by following the formation of the conjugated diene product at 234 nm (&... | Biochemistry 55: 3329-40 (2016) Article DOI: 10.1021/acs.biochem.6b00338 BindingDB Entry DOI: 10.7270/Q21N7ZXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||