

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM19145 (2S)-2-[(tert-butylcarbamothioyl)amino]-N-cyclopentyl-7-sulfanylheptanamide::thiolate analogue, 36a
SMILES: CC(C)(C)NC(=S)N[C@@H](CCCCCS)C(=O)NC1CCCC1
InChI Key: InChIKey=VXMHHEQJZJBMRW-AWEZNQCLSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone Deacetylase 6 (HDAC6) (Mus musculus) | BDBM19145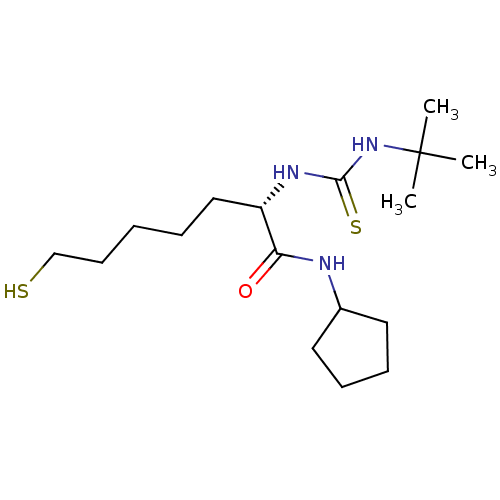 ((2S)-2-[(tert-butylcarbamothioyl)amino]-N-cyclopen...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Nagoya City University | Assay Description The enzyme activity was assayed using recombinant HDAC and [3H] acetyl-labeled histones as substrate. The released [3H]acetic acid was extracted and ... | J Med Chem 50: 5425-38 (2007) Article DOI: 10.1021/jm7009217 BindingDB Entry DOI: 10.7270/Q2XK8CT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19145 ((2S)-2-[(tert-butylcarbamothioyl)amino]-N-cyclopen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University | Assay Description The enzyme activity was assayed using recombinant HDAC and [3H] acetyl-labeled histones as substrate. The released [3H]acetic acid was extracted and ... | J Med Chem 50: 5425-38 (2007) Article DOI: 10.1021/jm7009217 BindingDB Entry DOI: 10.7270/Q2XK8CT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19145 ((2S)-2-[(tert-butylcarbamothioyl)amino]-N-cyclopen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University | Assay Description The enzyme activity was assayed using recombinant HDAC and [3H] acetyl-labeled histones as substrate. The released [3H]acetic acid was extracted and ... | J Med Chem 50: 5425-38 (2007) Article DOI: 10.1021/jm7009217 BindingDB Entry DOI: 10.7270/Q2XK8CT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||