

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM19189 7-(3-Chlorobenzyloxy)-4-carboxaldehyde-coumarin, 3::7-[(3-chlorophenyl)methoxy]-2-oxo-2H-chromene-4-carbaldehyde::C17
SMILES: Clc1cccc(COc2ccc3c(C=O)cc(=O)oc3c2)c1
InChI Key: InChIKey=ZOCADHRNWNJARU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM19189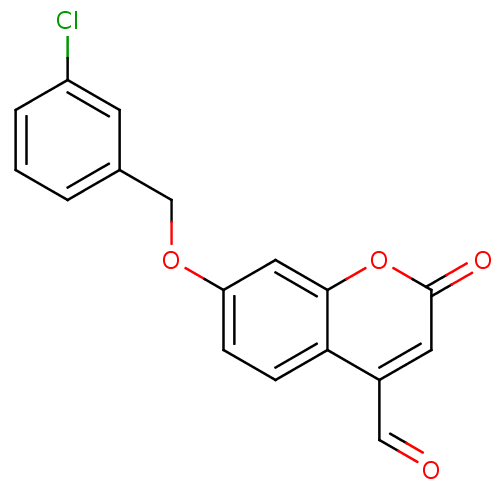 (7-(3-Chlorobenzyloxy)-4-carboxaldehyde-coumarin, 3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 400 | -8.72 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Pavia | Assay Description MAO B activities were determined spectrophotometrically at 250 nm using benzylamine as substrate. Competitive Ki values were determined by measuring ... | J Med Chem 50: 5848-5852 (2007) Article DOI: 10.1021/jm070677y BindingDB Entry DOI: 10.7270/Q2DN43B4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM19189 (7-(3-Chlorobenzyloxy)-4-carboxaldehyde-coumarin, 3...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10E+4 | -6.76 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Pavia | Assay Description MAO A activities were determined spectrophotometrically at 316 nm using kynuramine as substrate. Competitive Ki values were determined by measuring i... | J Med Chem 50: 5848-5852 (2007) Article DOI: 10.1021/jm070677y BindingDB Entry DOI: 10.7270/Q2DN43B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine oxidase (Rattus norvegicus (rat)) | BDBM19189 (7-(3-Chlorobenzyloxy)-4-carboxaldehyde-coumarin, 3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat MAO-B using Kynuramine as substrate assessed as formation of 4-hydroxyquinoline preincubated for 5 mins prior to sub... | Eur J Med Chem 70: 723-39 (2013) Article DOI: 10.1016/j.ejmech.2013.09.034 BindingDB Entry DOI: 10.7270/Q2WM1HCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Monoamine Oxidase Type B (MAO-B) (Rattus norvegicus (rat)) | BDBM19189 (7-(3-Chlorobenzyloxy)-4-carboxaldehyde-coumarin, 3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat MAO-B in brain mitochondrial homogenate assessed as 4-hydroxyquinoline by spectrophotometric method | Eur J Med Chem 89: 98-105 (2014) Article DOI: 10.1016/j.ejmech.2014.10.029 BindingDB Entry DOI: 10.7270/Q2FX7C3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||