

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM19206 (1S)-1-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4aH,5H,6H,7H,8H-cyclohexa[f]indazol-5-yl]but-3-en-1-ol::N-Arylpyrazolo[3,2-c]-Based Ligand, 30
SMILES: [H][C@@]1(CCCC2=Cc3c(C[C@]12C)cnn3-c1ccc(F)cc1)[C@@H](O)CC=C
InChI Key: InChIKey=QELYUNHSBLBUDL-HJNYFJLDSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19206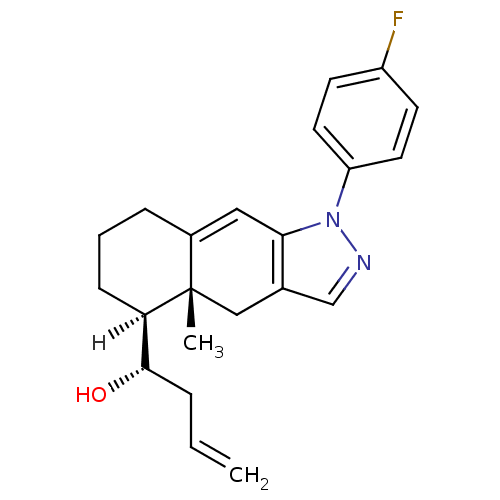 ((1S)-1-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19206 ((1S)-1-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of PMA-induced AP1 activity after 6 hrs by l... | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19206 ((1S)-1-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90 | n/a | 9.30 | n/a | n/a | 7.5 | 4 |
Merck Research Laboratories | Assay Description The IC50s were determined by incubating the receptors with radiolabeled dexamethasone in the presence of full log scale concentrations (10-11 M to 10... | J Med Chem 47: 2441-52 (2004) Article DOI: 10.1021/jm030585i BindingDB Entry DOI: 10.7270/Q28W3BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Homo sapiens (Human)) | BDBM19206 ((1S)-1-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to androgen receptor | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||