

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)CCC(=O)NC1CCN(CC1)c1ncnc2n(c(nc12)-c1ccccc1Cl)-c1ccc(Cl)cc1
InChI Key: InChIKey=OQJKJEJGASFVMR-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192537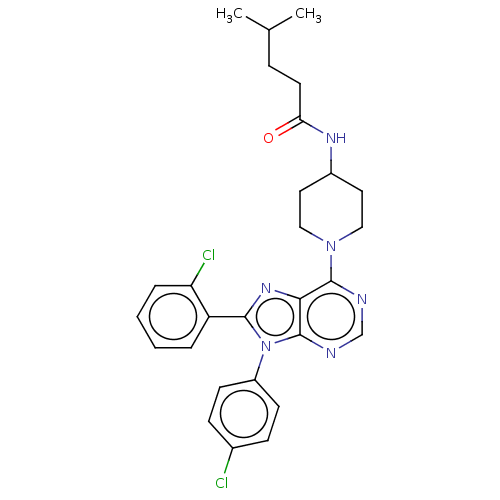 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192537 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 56: 8066-72 (2013) Article DOI: 10.1021/jm401129n BindingDB Entry DOI: 10.7270/Q2F192NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192537 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB2 receptor (unknown origin) expressed in CHO-K1 cells | J Med Chem 56: 8066-72 (2013) Article DOI: 10.1021/jm401129n BindingDB Entry DOI: 10.7270/Q2F192NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192537 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||