

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM193745 1-(3-((2-Hydroxy-6-oxocyclohex-1-en-1-yl)diazenyl)phenyl)-5-phenyl-N3,N4-bis(5-sulfamoyl-1,3,4-thiadiazol-2-yl)-1H-pyrazole-3,4-dicarboxamide (13)
SMILES: [#7]S(=O)(=O)c1nnc(-[#7]-[#6](=O)-c2nn(c(c2-[#6](=O)-[#7]-c2nnc(s2)S([#7])(=O)=O)-c2ccccc2)-c2cccc(-[#7]\[#7]=[#6]-3/[#6](=O)-[#6]-[#6]-[#6]-[#6]-3=O)c2)s1
InChI Key: InChIKey=RNXKCPNVYGFQOO-UHFFFAOYSA-N
Data: 2 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM193745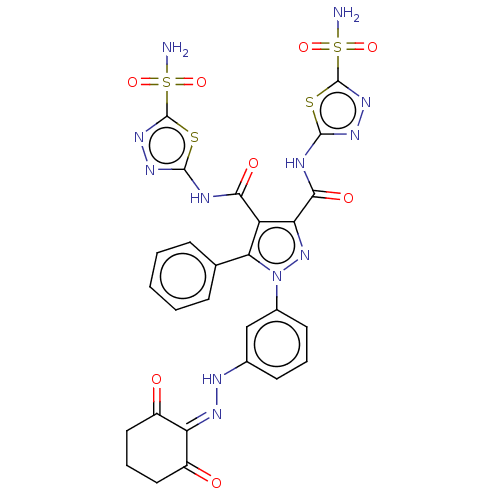 (1-(3-((2-Hydroxy-6-oxocyclohex-1-en-1-yl)diazenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description CA activity was measured according to the method described by Verpoorte et al. in inhibition studies, spectrophotometrically [Biochem., 242:4221-4229... | Bioorg Chem 68: 64-71 (2016) Article DOI: 10.1016/j.bioorg.2016.07.006 BindingDB Entry DOI: 10.7270/Q2N29VQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM193745 (1-(3-((2-Hydroxy-6-oxocyclohex-1-en-1-yl)diazenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 508 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description CA activity was measured according to the method described by Verpoorte et al. in inhibition studies, spectrophotometrically [Biochem., 242:4221-4229... | Bioorg Chem 68: 64-71 (2016) Article DOI: 10.1016/j.bioorg.2016.07.006 BindingDB Entry DOI: 10.7270/Q2N29VQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||