

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM194584 US9205085, MSX-207
SMILES: NCCCC[C@@H]1NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](CCCCN)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCCNC(N)=N
InChI Key: InChIKey=DGQKRQOCJFODHN-OIHVMPBRSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 4 (CXCR4/SDF-1) (Rattus norvegicus (Rat)) | BDBM194584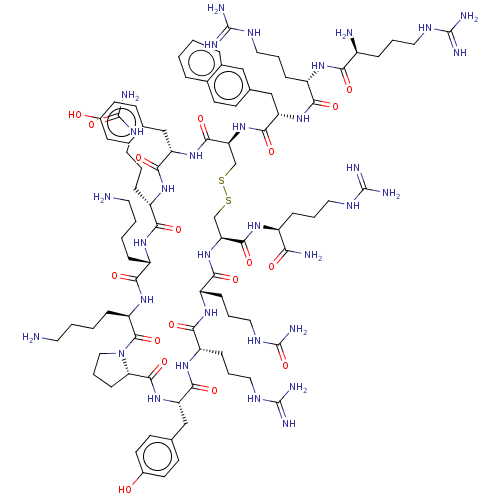 (US9205085, MSX-207) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University US Patent | Assay Description A synthetic 14-mer peptide, TN14003, was previously reported to block both SDF-1/CXCR4 mediated invasion in vitro and metastasis in vivo with a high ... | US Patent US9205085 (2015) BindingDB Entry DOI: 10.7270/Q2FF3R5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||