

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM19502 (2R)-N-(1-cyanocyclopropyl)-3-(cyclopropylmethane)sulfonyl-2-{[(1S)-2,2,2-trifluoro-1-(4-fluorophenyl)ethyl]amino}propanamide::trifluoroethylamine inhibitor, 12
SMILES: Fc1ccc(cc1)[C@H](N[C@@H](CS(=O)(=O)CC1CC1)C(=O)NC1(CC1)C#N)C(F)(F)F
InChI Key: InChIKey=NOTSCKFHTQJLMM-HOTGVXAUSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM19502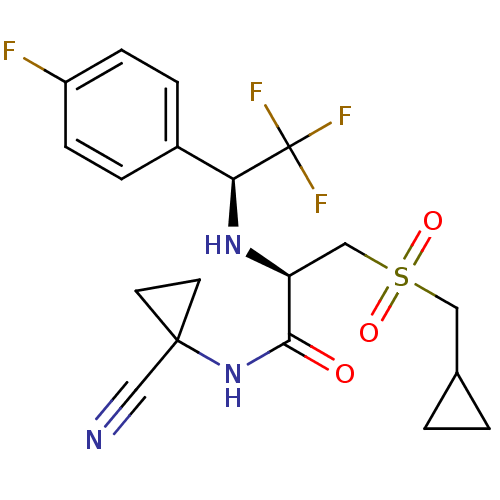 ((2R)-N-(1-cyanocyclopropyl)-3-(cyclopropylmethane)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 4929-33 (2007) Article DOI: 10.1016/j.bmcl.2007.06.023 BindingDB Entry DOI: 10.7270/Q26W98C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19502 ((2R)-N-(1-cyanocyclopropyl)-3-(cyclopropylmethane)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 4929-33 (2007) Article DOI: 10.1016/j.bmcl.2007.06.023 BindingDB Entry DOI: 10.7270/Q26W98C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19502 ((2R)-N-(1-cyanocyclopropyl)-3-(cyclopropylmethane)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 4929-33 (2007) Article DOI: 10.1016/j.bmcl.2007.06.023 BindingDB Entry DOI: 10.7270/Q26W98C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19502 ((2R)-N-(1-cyanocyclopropyl)-3-(cyclopropylmethane)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S-mediated antigen presentation in B/T hybridoma cells assessed as IL-2 level | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19502 ((2R)-N-(1-cyanocyclopropyl)-3-(cyclopropylmethane)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin L using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19502 ((2R)-N-(1-cyanocyclopropyl)-3-(cyclopropylmethane)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19502 ((2R)-N-(1-cyanocyclopropyl)-3-(cyclopropylmethane)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 4929-33 (2007) Article DOI: 10.1016/j.bmcl.2007.06.023 BindingDB Entry DOI: 10.7270/Q26W98C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||