

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM19545 (2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-4-methyl-2-[(3-methylphenyl)formamido]pentanamide::CHEMBL64765::arylaminoethyl amide, 1
SMILES: COc1ccc(NCCNC(=O)[C@H](CC(C)C)NC(=O)c2cccc(C)c2)cc1
InChI Key: InChIKey=BCHIMBJKORTZTR-NRFANRHFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Procathepsin L (Homo sapiens (Human)) | BDBM19545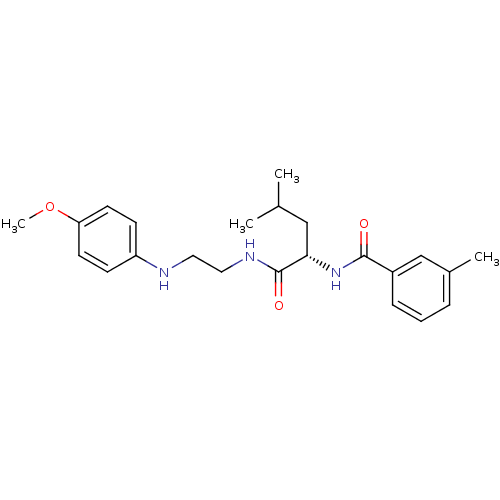 ((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 15: 4979-84 (2005) Article DOI: 10.1016/j.bmcl.2005.08.017 BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19545 ((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1486-90 (2006) Article DOI: 10.1016/j.bmcl.2005.12.056 BindingDB Entry DOI: 10.7270/Q2TQ5ZTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19545 ((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 15: 4979-84 (2005) Article DOI: 10.1016/j.bmcl.2005.08.017 BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19545 ((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | -10.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 15: 4979-84 (2005) Article DOI: 10.1016/j.bmcl.2005.08.017 BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19545 ((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 68 | -10.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1486-90 (2006) Article DOI: 10.1016/j.bmcl.2005.12.056 BindingDB Entry DOI: 10.7270/Q2TQ5ZTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19545 ((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1486-90 (2006) Article DOI: 10.1016/j.bmcl.2005.12.056 BindingDB Entry DOI: 10.7270/Q2TQ5ZTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19545 ((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human cathepsin S activity | Bioorg Med Chem Lett 13: 1997-2001 (2003) BindingDB Entry DOI: 10.7270/Q2VD6XTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19545 ((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human cathepsin K activity using fluorescence assay | Bioorg Med Chem Lett 13: 1997-2001 (2003) BindingDB Entry DOI: 10.7270/Q2VD6XTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19545 ((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <32 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human cathepsin L activity | Bioorg Med Chem Lett 13: 1997-2001 (2003) BindingDB Entry DOI: 10.7270/Q2VD6XTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||