

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM19614 (2S)-2-(1,3-benzoxazol-2-ylamino)-3-cyclohexyl-N-{2-[(4-methoxyphenyl)amino]ethyl}propanamide::Heterocyclic arylaminoethyl amide, 3
SMILES: COc1ccc(NCCNC(=O)[C@H](CC2CCCCC2)Nc2nc3ccccc3o2)cc1
InChI Key: InChIKey=VFNWTXUFNNOQHD-QFIPXVFZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM19614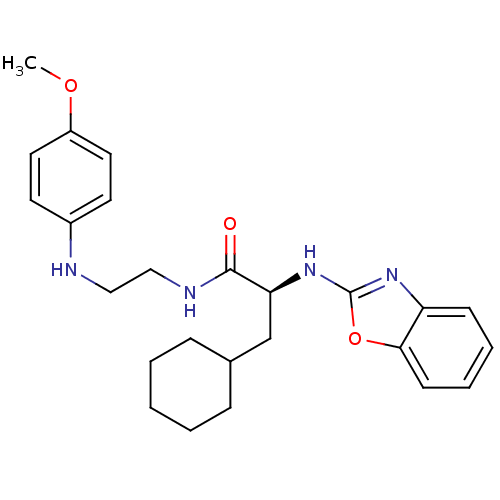 ((2S)-2-(1,3-benzoxazol-2-ylamino)-3-cyclohexyl-N-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | 29 | -10.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19614 ((2S)-2-(1,3-benzoxazol-2-ylamino)-3-cyclohexyl-N-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19614 ((2S)-2-(1,3-benzoxazol-2-ylamino)-3-cyclohexyl-N-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||