

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM199180 US9217012, 10
SMILES: CCc1ccc(cc1)C(=O)NCCCCC(NC(=O)C(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)COC1CC(C)CCC1C(C)C)C(N)=O
InChI Key: InChIKey=DZZMIHDOJVEPRL-UHFFFAOYSA-N
Data: 16 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM199180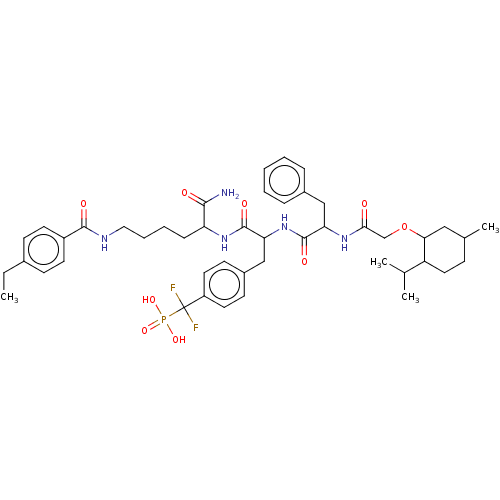 (US9217012, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.10 | -11.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.30 | -11.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 23.1 | -10.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 34 | -10.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase MEG2 (PTP-Meg2) (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-8.18 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 2A regulatory subunit B' (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-8.18 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase F (LAR) (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-8.18 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte common antigen (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-8.18 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase CDC14A (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-8.18 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase (VHR) (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-8.18 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase VHX (VHX) (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-8.18 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight phosphotyrosine protein phosphatase (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-8.18 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic cell protein-tyrosine phosphatase 70Z-PEP (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-8.18 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic protein-tyrosine phosphatase (HEPTP) (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-8.18 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-8.18 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fas-associated protein-tyrosine phosphatase 1 (FAP1) (Homo sapiens (Human)) | BDBM199180 (US9217012, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-8.18 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||