

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM201 (2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-acetamido-3-methylbutanehydrazido]-3-hydroxy-1-phenylbutan-2-yl]-2-acetamido-3-methylbutanamide::CGP 53820
SMILES: CC(C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(CC1CCCCC1)NC(=O)[C@@H](NC(C)=O)C(C)C
InChI Key: InChIKey=JNBVLGDICHLLTN-DZUOILHNSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM201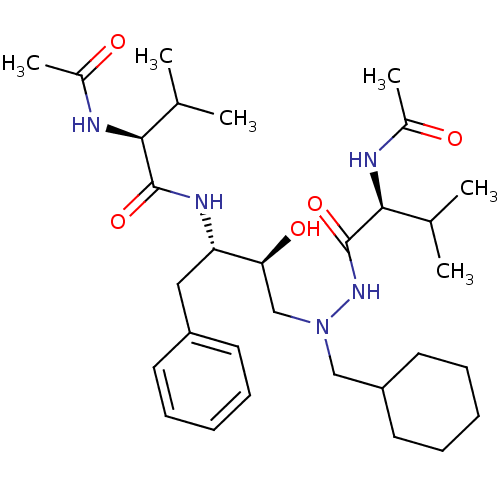 ((2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-ac...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Ciba-Geigy Ltd. | Assay Description A peptide cleavage assay was performed using the icosapeptide H-Arg-Arg-Ser-Asn-Gln-Val-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Asn-Ile-Gln-Gly-Arg-Arg-OH, a... | J Med Chem 39: 3203-16 (1996) Article DOI: 10.1021/jm960022p BindingDB Entry DOI: 10.7270/Q2FT8J7Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM201 ((2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-ac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against human cathepsin D | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM201 ((2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | >5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against human renin | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM201 ((2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-ac...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease at pH 6 using 164 microM peptide substrate | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pepsinogen C (Homo sapiens (Human)) | BDBM201 ((2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against human gastricsin | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM201 ((2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against human cathepsin E | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM201 ((2S)-N-[(2S,3S)-4-[(2S)-N'-(cyclohexylmethyl)-2-ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against human pepsin | Bioorg Med Chem Lett 3: 2837-2842 (1993) Article DOI: 10.1016/S0960-894X(01)80775-7 BindingDB Entry DOI: 10.7270/Q22F7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||