

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM20192 (1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-ol::cholesterol
SMILES: [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C
InChI Key: InChIKey=HVYWMOMLDIMFJA-DPAQBDIFSA-N
Data: 5 IC50 6 Kd 1 EC50 1 Other
PDB links: 407 PDB IDs match this monomer. 7 PDB IDs contain this monomer as substructures. 4 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20192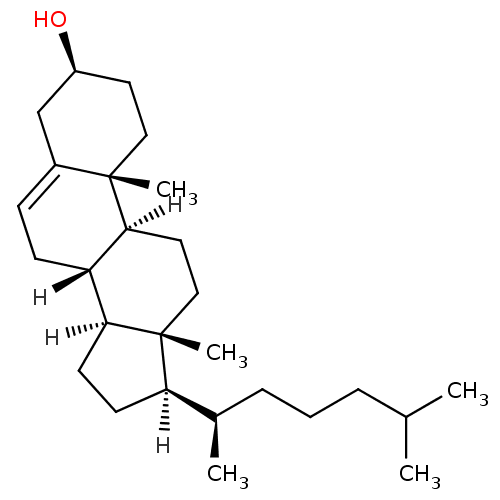 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 125 (CYP125) (Mycobacterium tuberculosis) | BDBM20192 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
Manchester Interdisciplinary Biocentre | Assay Description Substrate and ligand binding assay using UV- visible absorbance analysis of CYP142 was done on a Cary UV-50 UV-visible scanning spectrophotometer (Va... | J Biol Chem 285: 38270-82 (2010) Article DOI: 10.1074/jbc.M110.164293 BindingDB Entry DOI: 10.7270/Q2251GR0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cytochrome P450 142 (CYP142) (Mycobacterium tuberculosis) | BDBM20192 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a |
Manchester Interdisciplinary Biocentre | Assay Description Substrate and ligand binding assay using UV- visible absorbance analysis of CYP142 was done on a Cary UV-50 UV-visible scanning spectrophotometer (Va... | J Biol Chem 285: 38270-82 (2010) Article DOI: 10.1074/jbc.M110.164293 BindingDB Entry DOI: 10.7270/Q2251GR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan-rich sensory protein/translocator protein (TSPO) (Fremyella diplosiphon (Cyanobacterium)) | BDBM20192 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.75E+5 | n/a | n/a | n/a | n/a | n/a |
Michigan State University | Assay Description Tryptophan fluorescence measurements were performed with purified FdTSPO1 protein and ligand essentially as described by Li et al.; 2.5 μM prote... | Biochemistry 56: 73-84 (2017) Article DOI: 10.1021/acs.biochem.6b01019 BindingDB Entry DOI: 10.7270/Q2F47N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan-rich sensory protein/translocator protein (TSPO) (Fremyella diplosiphon (Cyanobacterium)) | BDBM20192 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 2.02E+5 | n/a | n/a | n/a | n/a | n/a |
Michigan State University | Assay Description Tryptophan fluorescence measurements were performed with purified FdTSPO1 protein and ligand essentially as described by Li et al.; 2.5 μM prote... | Biochemistry 56: 73-84 (2017) Article DOI: 10.1021/acs.biochem.6b01019 BindingDB Entry DOI: 10.7270/Q2F47N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| OSBP-related protein 4 (ORP4L) (Homo sapiens (Human)) | BDBM20192 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 68 | n/a | n/a | n/a | 7.4 | 20 |
Dalhousie University | Assay Description Recombinant OSBP, ORP4L, or ORP4S (8 pmol) was incubated in 75 μl of binding buffer (10 mM HEPES (pH 7.4), 150 mM KCl, 2% (w/v) polyvinyl alcohol)... | J Biol Chem 289: 15705-17 (2014) Article DOI: 10.1074/jbc.M114.571216 BindingDB Entry DOI: 10.7270/Q2N29VTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM20192 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Agonist activity at 6xHis tagged human RORgammat LBD (262 to 507 residues) expressed in Escherichia coli BL21 (DE3) assessed as biotinylated SRC1-2 p... | J Med Chem 61: 5794-5804 (2018) Article DOI: 10.1021/acs.jmedchem.7b01314 BindingDB Entry DOI: 10.7270/Q2M32Z85 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA polymerase (alpha/delta/epsilon) (Homo sapiens (Human)) | BDBM20192 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM20192 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM20192 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description TP_TRANSPORTER: increase in Daunorubicn intracellular accumulation (Daunorubicin: ? uM) in NIH-G185 cells | Biochem Biophys Res Commun 276: 909-16 (2000) Article DOI: 10.1006/bbrc.2000.3554 BindingDB Entry DOI: 10.7270/Q2862HR0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sterol 14α-demethylase (CYP51) (Homo sapiens (Human)) | BDBM20192 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ACT LLC Curated by ChEMBL | Assay Description Inhibition of human CYP51 expressed in Topp 3 cells by lanosterol demethylase assay | Drug Metab Dispos 35: 493-500 (2007) Article DOI: 10.1124/dmd.106.013888 BindingDB Entry DOI: 10.7270/Q2DF6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM20192 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >4.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant rat androgen receptor expressed in Escherichia coli using [3H]methyltrienolone (R 1881) | J Med Chem 48: 5666-74 (2005) Article DOI: 10.1021/jm050403f BindingDB Entry DOI: 10.7270/Q2TM7CBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| OSBP-related protein 4 (ORP4S) (Homo sapiens (Human)) | BDBM20192 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 60 | n/a | n/a | n/a | 7.4 | 20 |
Dalhousie University | Assay Description Recombinant OSBP, ORP4L, or ORP4S (8 pmol) was incubated in 75 μl of binding buffer (10 mM HEPES (pH 7.4), 150 mM KCl, 2% (w/v) polyvinyl alcohol)... | J Biol Chem 289: 15705-17 (2014) Article DOI: 10.1074/jbc.M114.571216 BindingDB Entry DOI: 10.7270/Q2N29VTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||