

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM20462 (5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]icosa-5,8,11,14-tetraenamide::Arachidonoyl dopamine::CHEMBL138921::N-arachidonoyl-dopamine (NADA)
SMILES: CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCc1ccc(O)c(O)c1
InChI Key: InChIKey=MVVPIAAVGAWJNQ-DOFZRALJSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM20462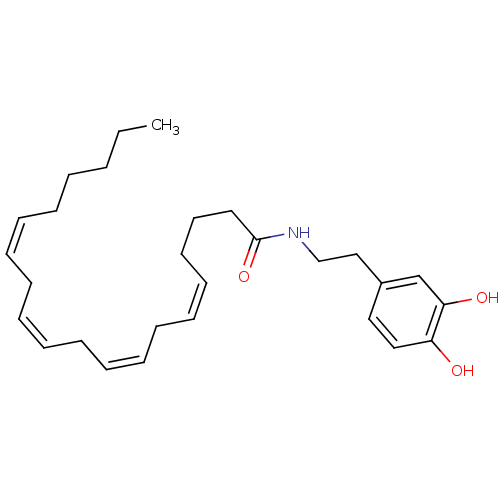 ((5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]ic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM20462 ((5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]ic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from rat brain CB1 receptor by liquid scintillation spectrophotometry | Cell Chem Biol 56: 8224-56 (2013) Article DOI: 10.1021/jm4005626 BindingDB Entry DOI: 10.7270/Q2B859M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM20462 ((5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]ic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS Curated by ChEMBL | Assay Description Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... | Bioorg Med Chem Lett 11: 447-9 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20462 ((5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]ic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | >6.31E+3 | >-7.09 | n/a | n/a | 1.48E+3 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM20462 ((5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]ic...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]Win55212-2 from CB2 receptor of rat spleen by liquid scintillation counting | Cell Chem Biol 56: 8224-56 (2013) Article DOI: 10.1021/jm4005626 BindingDB Entry DOI: 10.7270/Q2B859M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20462 ((5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]ic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Activation of human VR1 expressed in HEK293 cells assessed as stimulation of Ca2+ influx by Fluo-3 staining-based fluorescence assay | Cell Chem Biol 56: 8224-56 (2013) Article DOI: 10.1021/jm4005626 BindingDB Entry DOI: 10.7270/Q2B859M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||