

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM205095 US9556147, 4
SMILES: Cn1c2C[C@H](O)Cc3c(cccc3-n3ncc4cc(cc(F)c4c3=O)C(C)(C)C)-c2cc(Nc2ccc(cn2)C(=O)N2CCOCC2)c1=O
InChI Key: InChIKey=ACFBKRAWAGNCMP-XMMPIXPASA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205095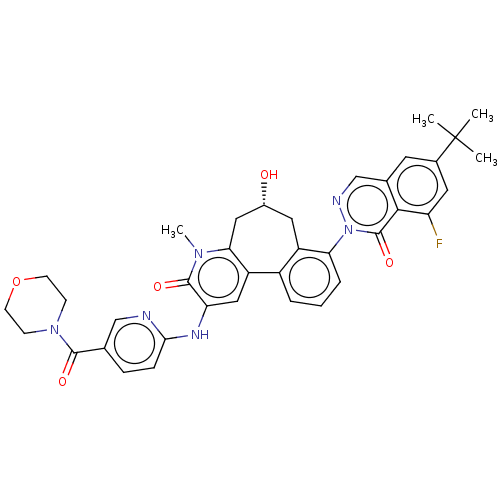 (US9556147, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205095 (US9556147, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of BTK in goat F(ab')2 anti-human IgM-stimulated human whole blood assessed as suppression of CD69 expression on B cells pretreated for 30... | Bioorg Med Chem Lett 29: 1074-1078 (2019) Article DOI: 10.1016/j.bmcl.2019.03.001 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205095 (US9556147, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of Kinase Tracer 178 binding to biotinylated-BTK (unknown origin) measured after 18 to 24 hrs by Europium-labeled Streptavidin conjugate b... | Bioorg Med Chem Lett 29: 1074-1078 (2019) Article DOI: 10.1016/j.bmcl.2019.03.001 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||