

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CO[C@H]1C=CC2C3Cc4ccc(O)c5OC1[C@]2(CCN3C)c45
InChI Key: InChIKey=FNAHUZTWOVOCTL-JTWAHMQWSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM208981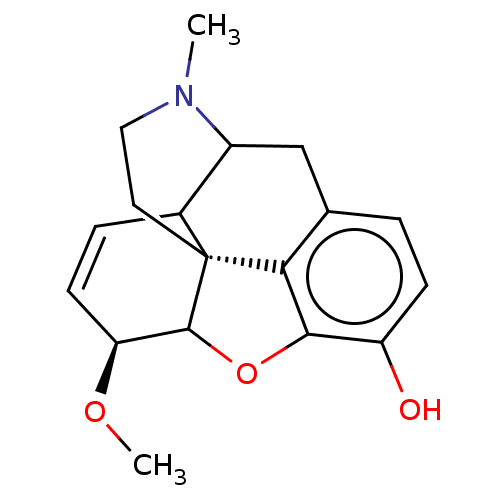 ((4R,4aR,7S,7aR,12bS)-3-methyl-2,3,4,4a,7,7a-hexahy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.44 | -11.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Nektar Therapeutics US Patent | Assay Description Briefly, serial dilutions of the test compounds were placed in a 96-well plate to which were added SPA beads, membrane and radioligand. The assay con... | US Patent US9233167 (2016) BindingDB Entry DOI: 10.7270/Q25T3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM208981 ((4R,4aR,7S,7aR,12bS)-3-methyl-2,3,4,4a,7,7a-hexahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 118 | -9.44 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Nektar Therapeutics US Patent | Assay Description Briefly, serial dilutions of the test compounds were placed in a 96-well plate to which were added SPA beads, membrane and radioligand. The assay con... | US Patent US9233167 (2016) BindingDB Entry DOI: 10.7270/Q25T3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM208981 ((4R,4aR,7S,7aR,12bS)-3-methyl-2,3,4,4a,7,7a-hexahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.30E+3 | -7.32 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Nektar Therapeutics US Patent | Assay Description Briefly, serial dilutions of the test compounds were placed in a 96-well plate to which were added SPA beads, membrane and radioligand. The assay con... | US Patent US9233167 (2016) BindingDB Entry DOI: 10.7270/Q25T3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM208981 ((4R,4aR,7S,7aR,12bS)-3-methyl-2,3,4,4a,7,7a-hexahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 624 | n/a | n/a | n/a | 25 |
Nektar Therapeutics US Patent | Assay Description Briefly, suspensions of cells expressing either the mu, kappa or delta opioid receptors were prepared in buffer containing 0.5 mM isobutyl-methyl xan... | US Patent US9233167 (2016) BindingDB Entry DOI: 10.7270/Q25T3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM208981 ((4R,4aR,7S,7aR,12bS)-3-methyl-2,3,4,4a,7,7a-hexahy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 28.5 | n/a | n/a | n/a | 25 |
Nektar Therapeutics US Patent | Assay Description Briefly, suspensions of cells expressing either the mu, kappa or delta opioid receptors were prepared in buffer containing 0.5 mM isobutyl-methyl xan... | US Patent US9233167 (2016) BindingDB Entry DOI: 10.7270/Q25T3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||