

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: NC(=O)[C@@H]1CCCCNC(=O)[C@@H](Cc2ccc(cc2)C(=O)c2ccccc2)NC(=O)[C@H](CC2CCCCC2)NC(=O)C[C@@H](O)[C@H](CC2CCCCC2)NC(=O)\C=C\C(=O)N1
InChI Key: InChIKey=ZUCWCMQJSSLFDY-HMJFQPJTSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM209027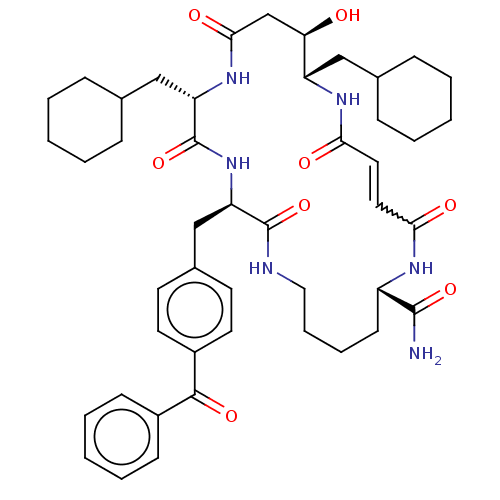 (US9243038, 3b (trans olefin)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
President and Fellows of Harvard College US Patent | Assay Description Macrocycles were assayed for IDE inhibition by monitoring cleavage of a substrate peptide containing a fluorophore-quencher pair such that cleavage o... | US Patent US9243038 (2016) BindingDB Entry DOI: 10.7270/Q2MW2FZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||