

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM209485 US10328074, Example III-26::US9266891, III-26
SMILES: CC(C)Nc1nc(cn2c(C)nnc12)-c1nc2ccc(cc2n1Cc1ccccn1)N1CCOCC1
InChI Key: InChIKey=CJHOZKNBDTVZRM-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRD4-BD1 and Histone H4 (Homo sapiens (Human)) | BDBM209485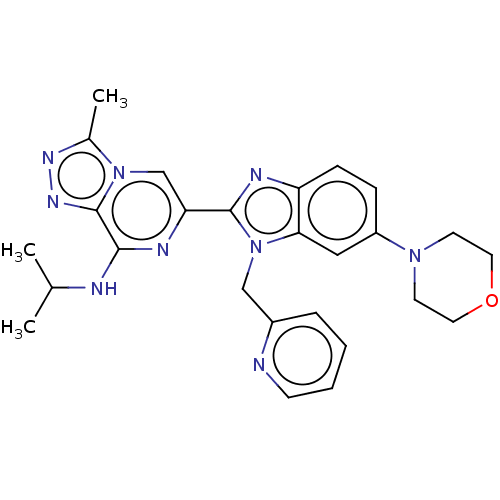 (US10328074, Example III-26 | US9266891, III-26) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds are diluted in serial dilution 1:5 in assay buffer from 10 mM stock in DMSO (100 uM start concentration) in white OptiPlate-384 (PerkinElme... | US Patent US9266891 (2016) BindingDB Entry DOI: 10.7270/Q23X85G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | US11077107, Example III-26 (US10328074, Example III-26 | US9266891, III-26) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM209485 (US10328074, Example III-26 | US9266891, III-26) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Displacement of biotinylated tetra-acetylated histone H4 peptide from GST-tagged BRD4-BD1 (44 to 168 residues) (unknown origin) measured after 30 min... | ACS Med Chem Lett 7: 728-9 (2016) Article DOI: 10.1021/acsmedchemlett.6b00259 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BRD4 BD1 (aa 42-168) (Homo sapiens (Human)) | BDBM209485 (US10328074, Example III-26 | US9266891, III-26) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description This assay is used to determine whether the compounds inhibit the interaction between the first (BRD4-BD1) or the second (BRD4-BD2) bromodomain of BR... | Bioorg Med Chem 17: 7324-36 (2009) BindingDB Entry DOI: 10.7270/Q20Z75M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||