

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM21009 C-terminal modified bifunctional peptide, 2::H-Tyr-D-Ala-Gly-Phe-Pro-Leu-Trp-O-Bzl::benzyl (2S)-2-[(2S)-2-{[(2S)-1-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]propanamido]acetamido}-3-phenylpropanoyl]pyrrolidin-2-yl]formamido}-4-methylpentanamido]-3-(1H-indol-3-yl)propanoate
SMILES: CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1ccccc1
InChI Key: InChIKey=AWLKNBWKVMBAKT-FDXPNMITSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21009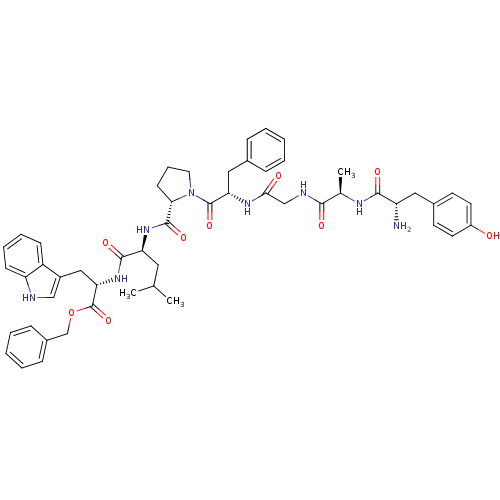 (C-terminal modified bifunctional peptide, 2 | H-Ty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 29 | -10.3 | n/a | n/a | 36 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21009 (C-terminal modified bifunctional peptide, 2 | H-Ty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 31 | -10.2 | n/a | n/a | 85 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurokinin 1 receptor (Homo sapiens (Human)) | BDBM21009 (C-terminal modified bifunctional peptide, 2 | H-Ty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | -9.54 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurokinin 1 receptor (Rattus norvegicus (rat)) | BDBM21009 (C-terminal modified bifunctional peptide, 2 | H-Ty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 270 | -8.95 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||