

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: NS(=O)(=O)c1ccc(cc1)-c1cnc2ccccc2c1
InChI Key: InChIKey=HGZKFXIUXSUJHM-UHFFFAOYSA-N
PDB links: 2 PDB IDs match this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM210938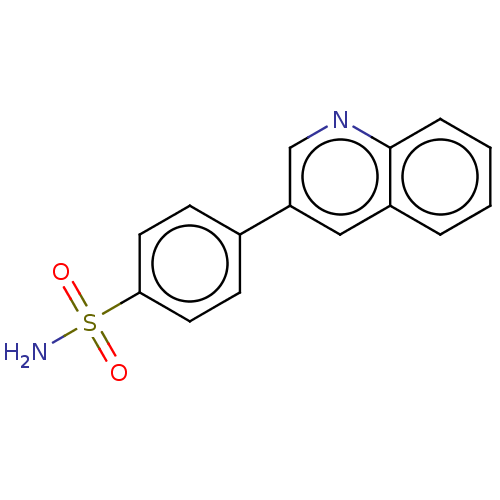 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM210938 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 [A65S,N67Q,E69T,I91L,F131V,K170E,L204A] (Homo sapiens (Human)) | BDBM210938 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM210938 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM210938 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM210938 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM210938 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM210938 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||