

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM21383 6-[4-(2-phenylphenyl)piperazin-1-yl]-N-(1,2,3,4-tetrahydronaphthalen-1-yl)hexanamide::NCGC00186032::Piperazinehexanamide derivative, 21
SMILES: O=C(CCCCCN1CCN(CC1)c1ccccc1-c1ccccc1)NC1CCCc2ccccc12
InChI Key: InChIKey=NMZIDFFHGCRAJV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotonin (5-HT) receptor (Rattus norvegicus (rat)) | BDBM21383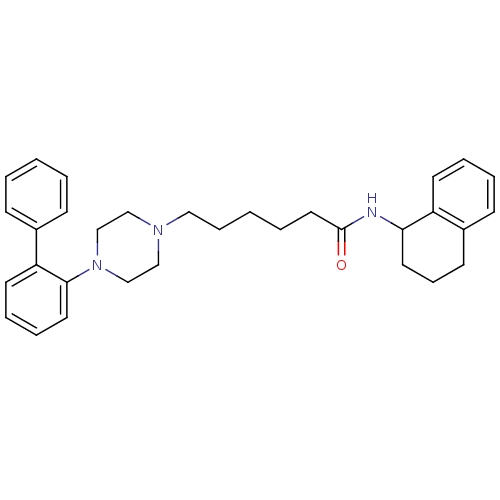 (6-[4-(2-phenylphenyl)piperazin-1-yl]-N-(1,2,3,4-te...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.130 | -14.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Universita degli Studi di Bari | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 4214-21 (2007) Article DOI: 10.1021/jm070487n BindingDB Entry DOI: 10.7270/Q20000CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM21383 (6-[4-(2-phenylphenyl)piperazin-1-yl]-N-(1,2,3,4-te...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 60.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 4214-21 (2007) Article DOI: 10.1021/jm070487n BindingDB Entry DOI: 10.7270/Q20000CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21383 (6-[4-(2-phenylphenyl)piperazin-1-yl]-N-(1,2,3,4-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 4214-21 (2007) Article DOI: 10.1021/jm070487n BindingDB Entry DOI: 10.7270/Q20000CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM21383 (6-[4-(2-phenylphenyl)piperazin-1-yl]-N-(1,2,3,4-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 4214-21 (2007) Article DOI: 10.1021/jm070487n BindingDB Entry DOI: 10.7270/Q20000CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-Lipoxygenase (LoxA) (Pseudomonas aeruginosa) | BDBM21383 (6-[4-(2-phenylphenyl)piperazin-1-yl]-N-(1,2,3,4-te...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
University of California, Santa Cruz | Assay Description Briefly, 3 uL of enzyme (approximately 20 nM LoxA, final concentration) or buffer (no-enzyme control) was dispensed into 1536-well Greiner black clea... | Biochemistry 55: 3329-40 (2016) Article DOI: 10.1021/acs.biochem.6b00338 BindingDB Entry DOI: 10.7270/Q21N7ZXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||