

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM21654 (2S)-2-[(2S)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-sulfanylpropanamido]-3-(1H-indol-3-yl)propanoic acid::Mercaptoacyl amino acid compound, 13c
SMILES: OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CS)[C@H]1CCc2ccccc12
InChI Key: InChIKey=AXECOZSOSVNVEZ-ZJOUEHCJSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21654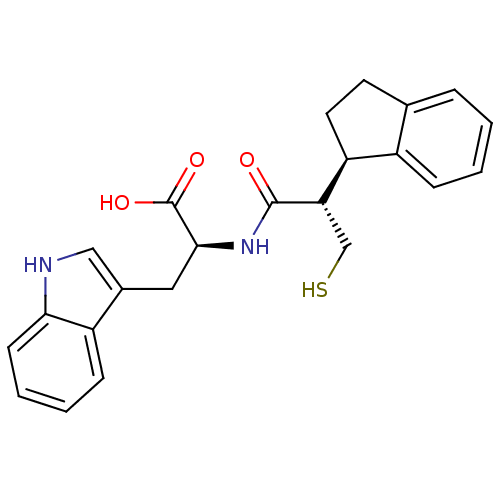 ((2S)-2-[(2S)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -12.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-Converting Enzyme (Rattus norvegicus) | BDBM21654 ((2S)-2-[(2S)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...) | KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (ECE1) (Homo sapiens (Human)) | BDBM21654 ((2S)-2-[(2S)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||