

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM21686 3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)uracil::6-[(3-ethyl-4-methylphenyl)amino]-3-(4-hydroxybutyl)-1,2,3,4-tetrahydropyrimidine-2,4-dione::CHEMBL327687::HB-EMAU::HBEMAU
SMILES: CCc1cc(Nc2cc(=O)n(CCCCO)c(=O)[nH]2)ccc1C
InChI Key: InChIKey=DKZBKKHAGYCGJG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA polymerase IIIC (Bacillus subtilis) | BDBM21686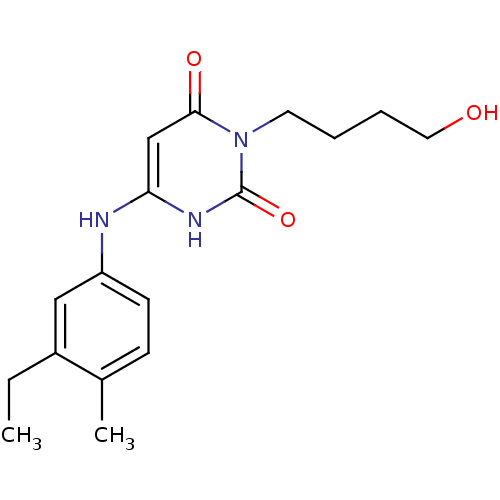 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Bacillus subtilis DNA polymerase IIIC using [3H]dTMP 250 pM (30 degree C for 10 min) | J Med Chem 48: 7063-74 (2005) Article DOI: 10.1021/jm050517r BindingDB Entry DOI: 10.7270/Q2W66MJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase IIIC (Bacillus subtilis) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Inhibitory activity against DNA polymerase IIIC from Bacillus subtilis was determined | J Med Chem 46: 2731-9 (2003) Article DOI: 10.1021/jm020591z BindingDB Entry DOI: 10.7270/Q2057GPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase IIIC (Bacillus subtilis) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 63 | -9.98 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase IIIC (Bacillus subtilis) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase IIIE (Bacillus subtilis) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.17E+5 | -5.45 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA Polymerase alpha (Bos taurus (bovine)) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.01E+6 | >-4.25 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase IIIE (Escherichia coli) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.01E+6 | >-4.15 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase gamma subunit 1 (Homo sapiens (Human)) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.01E+6 | >-4.25 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Microbiotix | Assay Description Reaction velocities were determined at each dGTP concentration and used to create double reciprocal plots of velocity versus dGTP concentration. The ... | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA Topoisomerase IV (Bacillus subtilis) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Microbiotix | Assay Description The 50% inhibitory concentration (IC50) is defined as the concentration of compound that inhibits the decatenation of kinetoplast DNA by 50%. | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA Gyrase (Bacillus subtilis) | BDBM21686 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Microbiotix | Assay Description The 50% inhibitory concentration (IC50) is defined as the concentration of compound that inhibits the decatenation of kinetoplast DNA by 50%. | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||