

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCn1cc(C(O)=O)c(=O)c2ccc(C)nc12
InChI Key: InChIKey=MHWLWQUZZRMNGJ-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA topoisomerase 4 subunit A [V210L]/B (Bacillus subtilis) | BDBM21691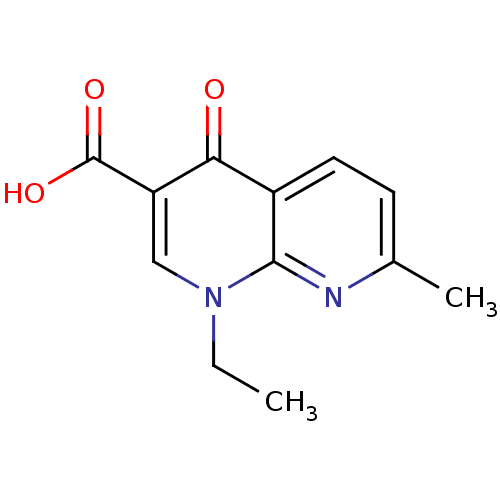 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.09E+6 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Microbiotix | Assay Description The 50% inhibitory concentration (IC50) is defined as the concentration of compound that inhibits the decatenation of kinetoplast DNA by 50%. | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyruvate kinase PKM (Homo sapiens (Human)) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter & Gamble Company US Patent | Assay Description A commercially available P450-GLO Assay kit (Promega Corporation, Madison Wis.) is used to screen various compounds for CYP3A4A inhibition activity. ... | US Patent US9138393 (2015) BindingDB Entry DOI: 10.7270/Q2GF0S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter & Gamble Company US Patent | Assay Description Cytochrome P450 is a large and diverse group of enzymes that catalyze the oxidation of organic substances. Some members of the CYP family contribute ... | US Patent US9144538 (2015) BindingDB Entry DOI: 10.7270/Q22806DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawai'i at Hilo Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase assessed as supercoiled plasmid DNA formation after 30 mins by electrophoretic analysis | J Med Chem 57: 8398-420 (2014) Article DOI: 10.1021/jm500853v BindingDB Entry DOI: 10.7270/Q25M679K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of DNA gyrase supercoiling in Escherichia coli. | Bioorg Med Chem Lett 8: 97-100 (1998) BindingDB Entry DOI: 10.7270/Q25141DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 2.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro antibacterial activity was determined as inhibitory concentration causing 50% DNA-gyrase supercoiling inhibition (SCI) | Citation and Details BindingDB Entry DOI: 10.7270/Q2FX7CM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ... | Toxicol Sci 136: 216-41 (2013) Article DOI: 10.1093/toxsci/kft176 BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 4 (Homo sapiens (Human)) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) Article DOI: 10.1093/toxsci/kft176 BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 3 (Homo sapiens (Human)) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ... | Toxicol Sci 136: 216-41 (2013) Article DOI: 10.1093/toxsci/kft176 BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 2 (Homo sapiens (Human)) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) Article DOI: 10.1093/toxsci/kft176 BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-beta (Homo sapiens (Human)) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of supercoiling activity of DNA gyrase in Escherichia coli | J Med Chem 40: 3292-6 (1997) Article DOI: 10.1021/jm9701583 BindingDB Entry DOI: 10.7270/Q2FJ2KHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A (Escherichia coli) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie-Paris 6 Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase GyrA/GyrB | Antimicrob Agents Chemother 52: 2909-14 (2008) Article DOI: 10.1128/AAC.01380-07 BindingDB Entry DOI: 10.7270/Q2862K8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.75E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie-Paris 6 Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis DNA gyrase GyrA/GyrB | Antimicrob Agents Chemother 52: 2909-14 (2008) Article DOI: 10.1128/AAC.01380-07 BindingDB Entry DOI: 10.7270/Q2862K8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase super-coiling activity using pBR322 DNA as substrate after 30 mins by fluorescence assay | Eur J Med Chem 174: 1-8 (2019) Article DOI: 10.1016/j.ejmech.2019.04.033 BindingDB Entry DOI: 10.7270/Q2TB1B6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Bacillus subtilis) | BDBM21691 (1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.49E+5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Microbiotix | Assay Description The 50% inhibitory concentration (IC50) is defined as the concentration of compound that inhibits the decatenation of kinetoplast DNA by 50%. | Antimicrob Agents Chemother 51: 119-27 (2007) Article DOI: 10.1128/AAC.01311-05 BindingDB Entry DOI: 10.7270/Q2988599 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||