

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM21791 2-[(1S)-5-{3-[4-(4-ethyl-1,3-thiazol-2-yl)-2-methoxyphenoxy]propoxy}-2,3-dihydro-1H-inden-1-yl]acetic acid::Indanylacetic Acid Analog, 34f
SMILES: CCc1csc(n1)-c1ccc(OCCCOc2ccc3[C@H](CC(O)=O)CCc3c2)c(OC)c1
InChI Key: InChIKey=YVTAAESLMBHKDQ-SFHVURJKSA-N
Data: 6 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM21791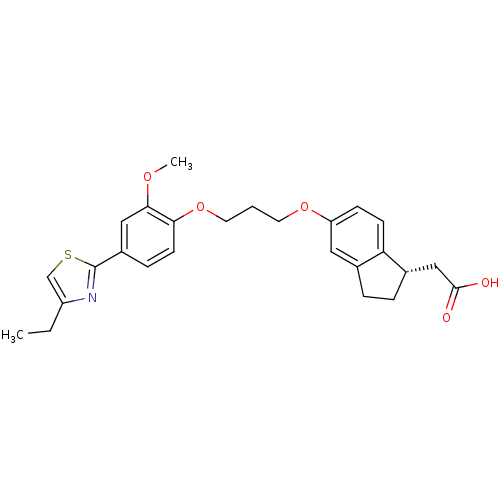 (2-[(1S)-5-{3-[4-(4-ethyl-1,3-thiazol-2-yl)-2-metho...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 254 | n/a | n/a | n/a | 25 |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Activity was assessed by measuring the human PPAR ligand binding domain with the co-activator protein. Europium labeled anti-GST antibody, streptavid... | J Med Chem 50: 984-1000 (2007) Article DOI: 10.1021/jm061299k BindingDB Entry DOI: 10.7270/Q2NK3C93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM21791 (2-[(1S)-5-{3-[4-(4-ethyl-1,3-thiazol-2-yl)-2-metho...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | 25 |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description Activity was assessed by measuring the human PPAR ligand binding domain with the co-activator protein. Europium labeled anti-GST antibody, streptavid... | J Med Chem 50: 984-1000 (2007) Article DOI: 10.1021/jm061299k BindingDB Entry DOI: 10.7270/Q2NK3C93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM21791 (2-[(1S)-5-{3-[4-(4-ethyl-1,3-thiazol-2-yl)-2-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 1.55E+7 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at Homo sapiens (human) PPARgamma expressed in mouse 3T3-L1 cells incubated for 2 days followed by compound wash out measured after ... | Citation and Details Article DOI: 10.1007/s00044-011-9599-z BindingDB Entry DOI: 10.7270/Q22J6FR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM21791 (2-[(1S)-5-{3-[4-(4-ethyl-1,3-thiazol-2-yl)-2-metho...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 3.94E+6 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at GST-tagged Homo sapiens (human) PPARalpha ligand binding domain after 2 hr by FRET assay | Citation and Details Article DOI: 10.1007/s00044-011-9599-z BindingDB Entry DOI: 10.7270/Q22J6FR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM21791 (2-[(1S)-5-{3-[4-(4-ethyl-1,3-thiazol-2-yl)-2-metho...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 4.00E+8 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at GST-tagged Homo sapiens (human) PPARdelta ligand binding domain after 2 hr by FRET assay | Citation and Details Article DOI: 10.1007/s00044-011-9599-z BindingDB Entry DOI: 10.7270/Q22J6FR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM21791 (2-[(1S)-5-{3-[4-(4-ethyl-1,3-thiazol-2-yl)-2-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | 25 |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description PPAR gamma activity was assessed by using the IRBA in mouse 3T3-L1 cells. This assay measures the ability of test compound to cause an increase in th... | J Med Chem 50: 984-1000 (2007) Article DOI: 10.1021/jm061299k BindingDB Entry DOI: 10.7270/Q2NK3C93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||