

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CS(=O)(=O)c1cccc(c1)-c1cc(NC2CC2)n2ncc(\C=C3/NC(=O)NC3=O)c2n1
InChI Key: InChIKey=LYSQLMKWDVAPMI-PXNMLYILSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Casein kinase II subunit alpha/beta (Homo sapiens (Human)-Rattus norvegicus) | BDBM218917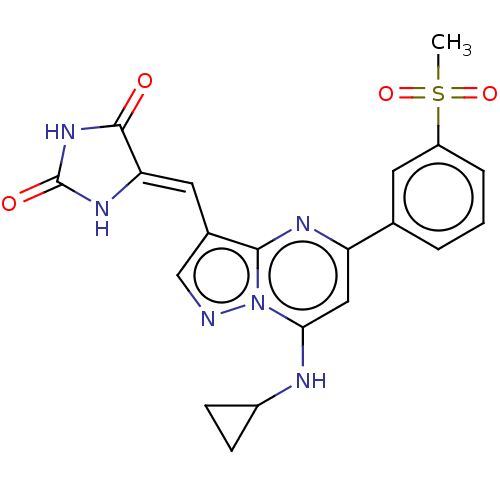 (US9303033, J7, Table 22A, Compound 4 | US9303033, ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.2 | n/a |
SENHWA BIOSCIENCES, INC. US Patent | Assay Description In a final reaction volume of 50 μl, CK2 ααββ (4 ng, 8.5 mU) was incubated with various concentrations of test compounds in ... | US Patent US9303033 (2016) BindingDB Entry DOI: 10.7270/Q2CN72RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit beta (Homo sapiens (Human)) | BDBM218917 (US9303033, J7, Table 22A, Compound 4 | US9303033, ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 649 | n/a | n/a | n/a | n/a | 7.0 | n/a |
SENHWA BIOSCIENCES, INC. US Patent | Assay Description 5 uM ATP: Test compounds dissolved and diluted in DMSO (2 μl) were added to a reaction mixture comprising 10 μl of 5× Reaction Buffer (40 m... | US Patent US9303033 (2016) BindingDB Entry DOI: 10.7270/Q2CN72RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit beta (Homo sapiens (Human)) | BDBM218917 (US9303033, J7, Table 22A, Compound 4 | US9303033, ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
SENHWA BIOSCIENCES, INC. US Patent | Assay Description 5 uM ATP: Test compounds dissolved and diluted in DMSO (2 μl) were added to a reaction mixture comprising 10 μl of 5× Reaction Buffer (40 m... | US Patent US9303033 (2016) BindingDB Entry DOI: 10.7270/Q2CN72RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha/beta (Homo sapiens (Human)-Rattus norvegicus) | BDBM218917 (US9303033, J7, Table 22A, Compound 4 | US9303033, ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.2 | n/a |
SENHWA BIOSCIENCES, INC. US Patent | Assay Description In a final reaction volume of 50 μl, CK2 ααββ (4 ng, 8.5 mU) was incubated with various concentrations of test compounds in ... | US Patent US9303033 (2016) BindingDB Entry DOI: 10.7270/Q2CN72RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||