

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM220664 US10633379, Compound Y::US9296741, 253
SMILES: CCNC(=O)c1cc2c(cn(C)c(=O)c2[nH]1)-c1cc(ccc1Oc1ccc(F)cc1F)S(C)(=O)=O
InChI Key: InChIKey=GLPRVXBSUHOVMP-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bromodomain-containing protein 4 (BRD4)(aa 352-457) (Homo sapiens (Human)) | BDBM220664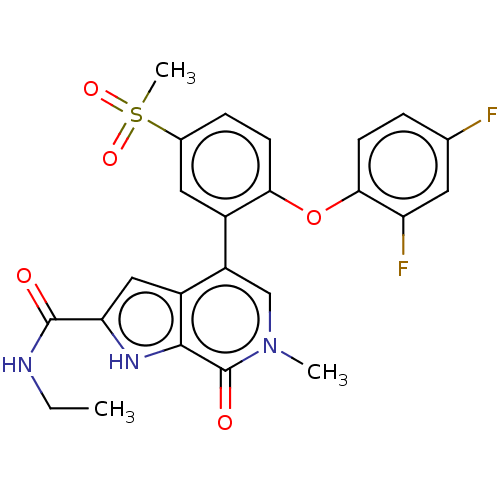 (US10633379, Compound Y | US9296741, 253) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 1.20 | -12.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220664 (US10633379, Compound Y | US9296741, 253) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (BRD4)(aa 352-457) (Homo sapiens (Human)) | BDBM220664 (US10633379, Compound Y | US9296741, 253) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description Compound dilution series were prepared in DMSO via an approximately 3-fold serial dilution. Compound dilutions were added directly into white, low-vo... | US Patent US10633379 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (BRD4)(aa 57-168) (Homo sapiens (Human)) | BDBM220664 (US10633379, Compound Y | US9296741, 253) | PDB GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 20.4 | -10.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220664 (US10633379, Compound Y | US9296741, 253) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD1 (57 to 168) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (BRD4)(aa 57-168) (Homo sapiens (Human)) | BDBM220664 (US10633379, Compound Y | US9296741, 253) | PDB GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 69.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description Compound dilution series were prepared in DMSO via an approximately 3-fold serial dilution. Compound dilutions were added directly into white, low-vo... | US Patent US10633379 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||