

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM22107 7-{[(2R,4R)-2,4-bis(hydroxymethyl)azetidin-1-yl]methyl}-3H,4H,5H-pyrrolo[3,2-d]pyrimidin-4-one::Azetidine based compound, (+/-) 41
SMILES: OC[C@H]1C[C@H](CO)N1Cc1c[nH]c2c1nc[nH]c2=O
InChI Key: InChIKey=ZZDOJITUEXBERC-RKDXNWHRSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22107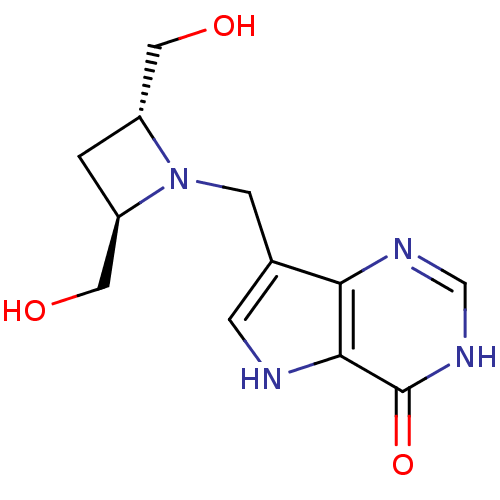 (7-{[(2R,4R)-2,4-bis(hydroxymethyl)azetidin-1-yl]me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 280 | -8.84 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine Nucleoside Phosphorylase (PNP) (Bos taurus (bovine)) | BDBM22107 (7-{[(2R,4R)-2,4-bis(hydroxymethyl)azetidin-1-yl]me...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 360 | -8.70 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine Nucleoside Phosphorylase (PNP) (Plasmodium falciparum) | BDBM22107 (7-{[(2R,4R)-2,4-bis(hydroxymethyl)azetidin-1-yl]me...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 580 | -8.42 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||