

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM223287 (1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-fluoro-2-methylpropyl)amino]-3-methoxypyrazin-2-yl}carbonyl)cyclopropanecarboxylic acid::US20160326143, 62::US9657001, 62
SMILES: COc1nc(cnc1C(=O)[C@H]1C[C@@H]1C(O)=O)N(CC(C)(C)F)c1cc(Cl)c(F)cc1F
InChI Key: InChIKey=RVMWIVNHZMXEHS-UWVGGRQHSA-N
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287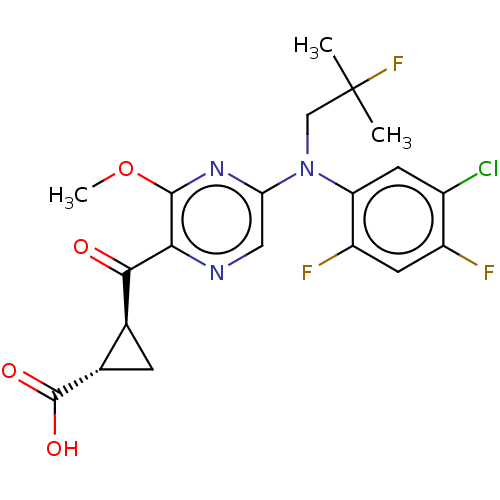 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.289 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astrazeneca Ab US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester. In order to obtain IC50-v... | US Patent US20160326143 (2016) BindingDB Entry DOI: 10.7270/Q2W66JN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.289 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester.In order to obtain IC50-va... | US Patent US9657001 (2017) BindingDB Entry DOI: 10.7270/Q2SJ1NPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP2D6 using coumarin based substrate by fluorescence assay | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP2C9 expressed in Escherichia coli using coumarin based substrate by fluorescence assay | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP2C19 using coumarin based substrate by fluorescence assay | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A4 expressed in Escherichia coli using coumarin based substrate by fluorescence assay | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A2 using coumarin based substrate by fluorescence assay | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||